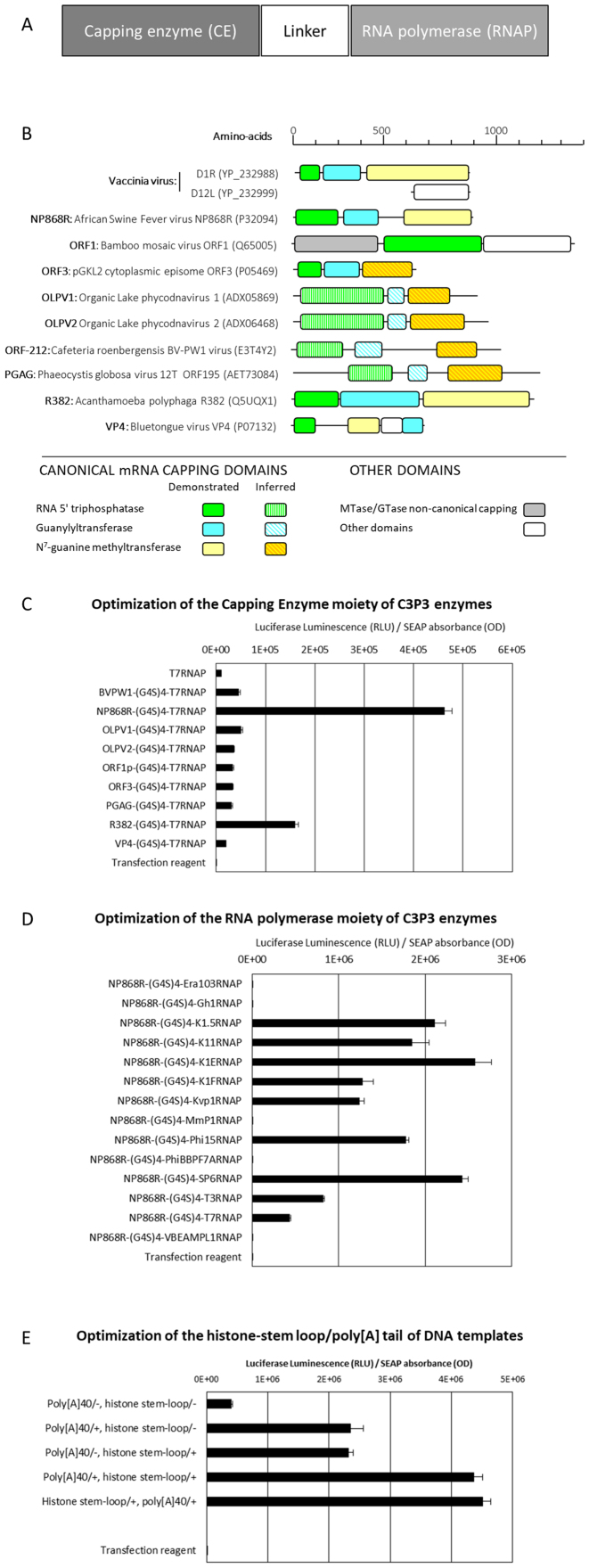
Figure 3.
Design and optimization of single-unit C3P3-G1 enzymes. (A) General design of the C3P3-G1 enzymes. Single-unit C3P3-G1 enzyme consists of three main domains (i.e. capping enzyme, linker and DNA-dependent RNA polymerase) which were optimized step-by-step in HEK-293 cells (similar results were obtained in CHO-K1 cells, data not shown). (B) Enzymatic domains of the candidate capping enzymes selected to generate C3P3-G1 enzymes. Capping enzymes were selected by bioinformatic sequence analysis showing that the proteins contain the three-enzymatic domains required for mRNA capping (i.e. TPase, GTase and N7-MTase). Blocks stand for different domains and their annotations are listed at the bottom of the figure. The lengths of the blocks are directly proportional to their sequence length. The enzymatic domains of the well-characterized heterodimeric vaccinia virus capping enzyme are also shown. (C) Selection of the mRNA capping moieties of C3P3-G1 enzymes. mRNA capping moieties were fused in-frame to the amino-terminal extremity of the T7RNAP sequence via (G4S)4 linker. Reverse constructions by in-frame fusion to the carboxyl-terminus of T7RNAP were not tested because T7RNAP does not tolerate carboxyl-terminal extensions (85,86). Constructions were tested for firefly luciferase reporter expression assay 24 h after transfection and expressed in RLU normalized for hSEAP expression expressed in DO (RLU/DO ratio). The errors bars indicate the standard deviation of at least four experiments. Comparisons of NP868R-(G4S)4-T7RNAP versus all other C3P3-G1 enzymes: P< 0.05, Student's t-test. (D) Selection of the phage DNA-dependent RNAPs moieties of C3P3-G1 enzymes. Phage DNA-dependent RNAPs were fused in-frame to the carboxyl-terminal extremity of the NP868R sequence via (G4S)4 flexible linker and tested for firefly luciferase reporter expression as described above. Comparisons of NP868R-(G4S)4-K1ERNAP versus NP868R-(G4S)4-K1.5RNAP and NP868R-(G4S)4-SP6RNAP: NS; NP868R-(G4S)4-K1ERNAP versus all other constructions: P< 0.05, Student's t-test. (E) Optimization of the polyadenylation/histone stem–loop region of DNA templates. A track of 40 adenosine residues of pK1E-Luciferase, which provides artificial polyadenylation to the transcripts was either removed or associated to the consensus stem-loop from human histone. This RNA element is involved in the regulation of stability and of translation efficiency in the cytoplasm and is functionally similar to a poly(A) tail in that it enhances translational efficiency and is dependent on mRNA capping. The corresponding pK1E-Luciferase with various sequence modifications were co-transfected with the pCMV-NP868R-(G4S)2-K1ERNAP(R551S) plasmid in HEK-293 cells. Comparison of poly[A40] vs. histone tem-loop: NS; poly[A40] versus no poly[A] track or poly[A] track placed before or after consensus stem-loop of human histone: P< 0.05, Student's t-test.