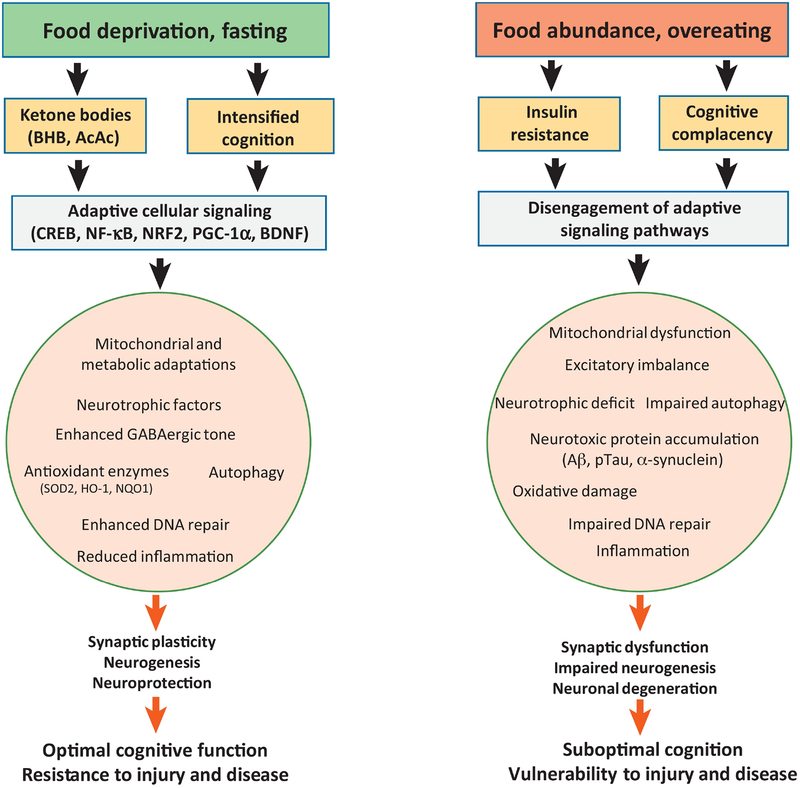
Figure 1. Cellular and molecular mechanisms by which food intake impacts neuroplasticity and cognition.
Left) Adaptive responses of neuronal networks to intermittent food deprivation or fasting. Extended periods with no or little energy intake triggers a metabolic shift from utilization of liver glycogen-derived glucose to adipose cell-derived fatty acids and ketone bodies (BHB, β-hydroxybutyrate; AcAc, acetoacetate) generated therefrom. In addition to serving as a source of acetyl CoA for mitochondrial ATP production, ketone bodies can activate signaling pathways involved in synaptic plasticity and cellular stress resistance, including those involving the transcription factors CREB (cyclic AMP response element binding protein) and NF-κB (nuclear factor kappa B), and neurotrophic factors such as BDNF (brain-derived neurotrophic factor). The increased activity in neuronal networks involved in cognitive processing during food seeking (navigation, decision-making, etc.) engages adaptive signaling pathways that bolster mitochondrial function and up-regulate neurotrophic factors, GABAergic tone, antioxidant defenses and DNA repair, while suppressing inflammation. These adaptive responses promote synaptic plasticity, neurogenesis and cellular stress resistance which, in turn enhances cognition and resistance of the brain to injury and disease. Right) Excessive food intake as occurs in laboratory animals fed ad libitum and most humans in modernized countries impairs neuroplasticity. Consumption of food throughout the waking hours results in little or no metabolic switching which can result in insulin resistance and a relative lack of engagement of neuronal networks involved in navigation and critical decision-making. As a consequence, signaling pathways that promote neuroplasticity and resilience are disengaged with the result being suboptimal cognitive abilities and vulnerability of the brain to stress and neurodegenerative disorders. Animal studies have shown that high energy diets and diabetes accelerate cognitive decline and motor dysfunction in models of Alzheimer’s disease (AD) and Parkinson’s disease (PD), respectively. Excessive energy intake accelerates the underlying accumulation of amyloid β-peptide (Aβ) and hyperphosphorylated Tau (pTau) in the brain in AD, and α-synuclein in PD. NRF2, nuclear regulatory factor 2; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α.