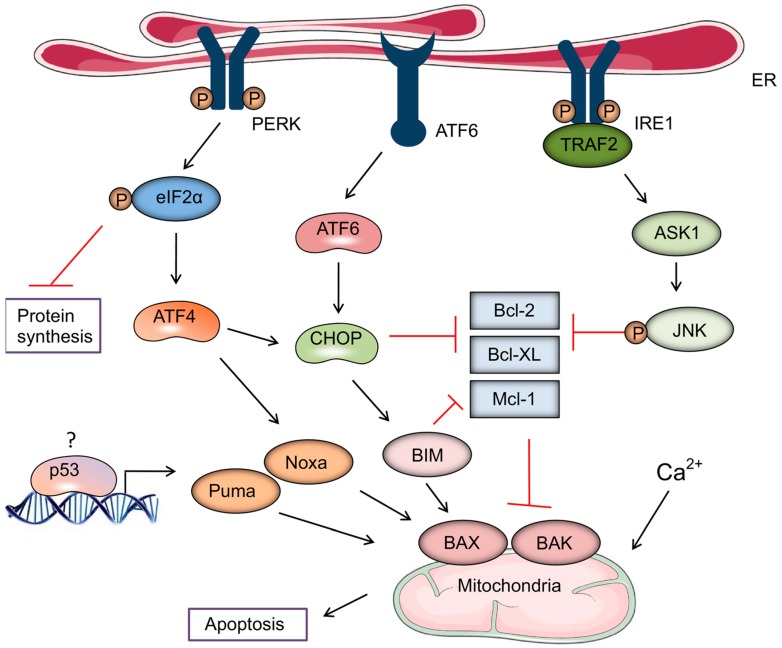
Figure 2.
Stress-induced cell death. Under endoplasmic reticulum (ER) stress, protein kinase RNA-like endoplasmic reticulum kinase (PERK) is activated and phosphorylates and inactivates eukaryotic initiation factor 2α (eIF2α). This results in the selective induction of activating transcription 4 (ATF4) and its downstream proteins C/EBP-homologous protein (CHOP) and Noxa, resulting in cell death. CHOP, which can also be induced by ATF6, induces Bim and inhibits Bcl-2. It is still not clear how p53 is induced under ER stress and induces Noxa and Puma, resulting in apoptosis. Inositol-requiring enzyme 1 (IRE1α) can recruit TNF receptor-associated factor 2 (TRAF2), which activates apoptosis signal-regulating kinase 1 (ASK1) and its downstream target c-Jun N-terminal kinase 1 (JNK). JNK can induce apoptosis by inhibiting anti-apoptotic proteins such as Bcl-2 and Bcl-xL. Reproduced with the permission of the authors, editor-in-chief and publisher (Wiley): Iurlaro; Muñoz-Pinedo. “Cell death induced by endoplasmic reticulum stress.” FEBS J. 2016, 283, 2640–2652, doi:10.1111/febs.13598. PMID: 26587781 (Reference [41] in the text).