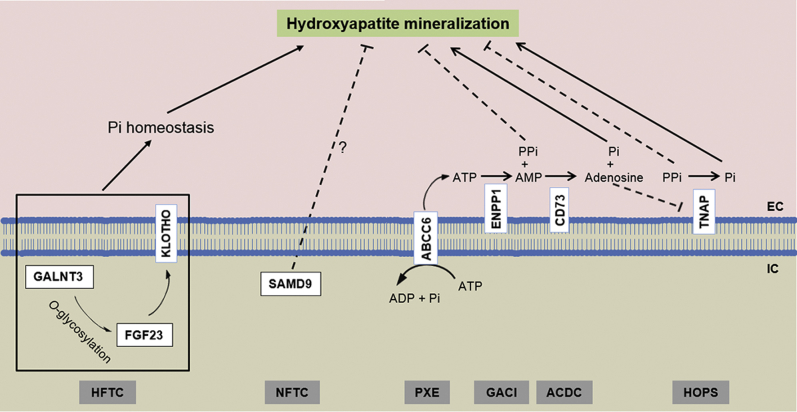
Figure 2.
Different pathomechanisms controlling ectopic mineralization. Hyperphosphatemic familial tumoral calcinosis (HFTC) is associated with altered Pi homeostasis (marked hyperphosphatemia) because of mutations in one of the three proteins involved in the regulation of inorganic phosphate (Pi) excretion in the kidney (GALNT3, FGF23, and KLOTHO). Normophosphatemic familial tumoral calcinosis (NFTC) is caused by mutations in the SAMD9 gene with unknown function in prevention of ectopic mineralization without altered serum phosphate levels. ATP-binding cassette, subfamily C, member 6 (ABCC6), ectonucleotide pyrophosphotase/phosphodiesterase 1 (ENPP1), and 5’-ecto nucleotidase (CD73) are plasma membrane–associated proteins controlling the synthesis and degradation of inorganic pyrophosphate (PPi). ENPP1 is the principal enzyme that generates PPi from ATP hydrolysis. ABCC6 works upstream of ENPP1 by mediating release of ATP, a substrate for ENPP1. CD73 cleaves AMP to adenosine and Pi, with adenosine being an inhibitor of tissue nonspecific alkaline phosphatase (TNAP), which degrades PPi. Mutations in these proteins result in pseudoxanthoma elasticum (PXE), generalized arterial calcification of infancy (GACI), and arterial calcification due to deficiency of CD73 (ACDC), respectively, all characterized by reduced plasma PPi levels. Loss-of-function mutations in the gene encoding TNAP, which degrades PPi to Pi, result in hypophosphatasia (HOPS) attributable to increased plasma PPi levels. Adapted with permission from Uitto et al.3 EC, extracellular; IC, intracellular.