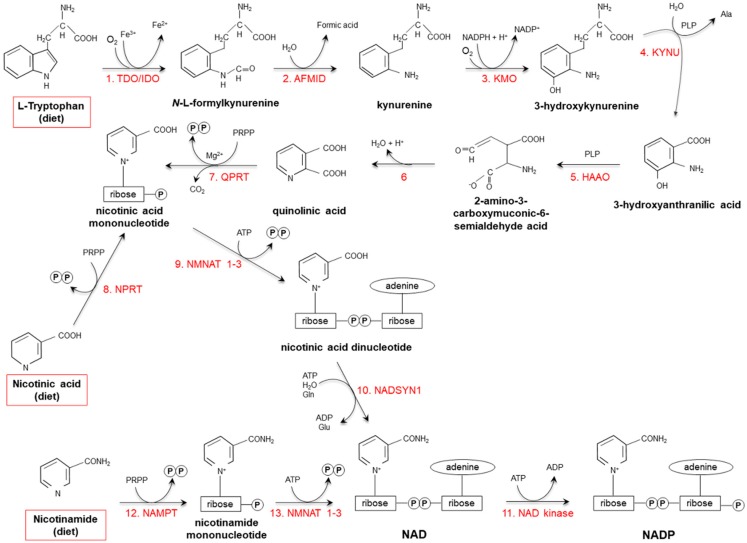
Figure 2.
De novo synthesis of NAD(P) from tryptophan, nicotinamide and nicotinic acid. (1) Two iron porphyrin metalloproteins, tryptophan 2,3 dioxygenase (TDO, in the liver) and indolamine-pyrrole 2-3 dioxygenase (IDO, in extrahepatic tissues), oxidize the pyrrole moiety of Tryptophan (Trp), thus forming N-L-formylkynurenine. (2) Arylformamidase (AFMID) hydrolytically removes the formyl group producing kynurenine and is then (3) hydroxylated to 3-hydroxykynurenine by kynurenine-3 monooxygenase (KMO), a mitochondrial flavo-enzyme that uses O2 as a substrate and NADPH as a cofactor. The action of (4) kynureninase B (KYNU, a vitamin B6-dependent enzyme) and (5) 3-hydroxyanthranilic dioxygenase (HAAO, a nonheme iron-dependent dioxygenase) leads to production of 2-amino-3-carboxymuconic-6-semialdehyde acid, an unstable product that (6) spontaneously condensates and rearranges to form quinolinic acid; then, (7) quinolinic acid is decarboxylated and converted to nicotinic acid mononucleotide by quinolinic acid phosphoribosyltransferase (QPRT). Nicotinic acid mononucleotide is also produced through the “salvage pathway”, via the action of (8) nicotinic acid phosphoribosyltransferase (NPRT). The subsequent action of (9) nicotinamide/nicotinic acid-mononucleotide-adenylyltransferases (NMNAT1-3) and (10) NAD synthetase (NADSYN1) leads to the generation of NAD, which is then (11) phosphorylated to produce NADP. NAD can also derive directly from nicotinamide through the action of (12) nicotinamide phosphoribosyltransferase (NAMPT) and (13) nicotinamide/nicotinic acid-mononucleotide-adenylyltransferase (NMNAT1-3). Red frames: dietary precursors of NAD(P). Ala: alanine; Gln: glutamine; Glu: glutamate; PLP pyridoxal phosphate; PRPP: 5-phosphoribosyl-1- pyrophosphate.