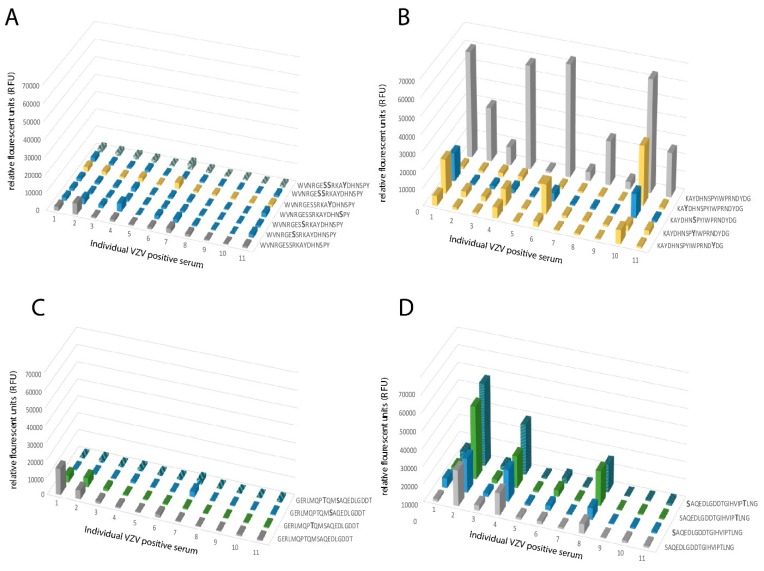
Figure 5.
A homogenous response towards synthetic peptides with single or multiple GalNAc modifications was observed for peptides covering parts of the N-terminal region of gE. (A) Synthetic peptide 64-WVNRGESSRKAYDHNSPY-81 unmodified or modified by GalNAc residues at either Ser or Tyr, or a combination of both. (B) Synthetic peptide 73-KAYDHNSPYIWPRNDYDG-90 unmodified or modified by GalNAc residues at either Ser or Tyr. (C) Synthetic peptide 111-GERLMQPTQMSAQEDLGDDT-130 unmodified or modified by GalNAc residues at either Ser or Thr, or a combination of both. (D) Synthetic peptide 121-SAQEDLGDDTGIHVIPTLNG-130 unmodified or modified by GalNAc residues at either Ser or Thr. All peptides were incubated with serum samples and the relative fluorescence unit (RFU)-values were determined. Grey: unglycosylated peptide. Blue: GalNAc modification on a Ser residue. Yellow: GalNAc modification on a Tyr residue. Green: GalNAc modification on a Thr residue. Blue and yellow: GalNAc modification on Ser and Tyr residues. Blue and green: GalNAc modification on Ser and Thr residues. X-axis shows the individual serum samples (n = 11) from VZV IgG-positive patients, plotted in the same order of individual sera in all graphs.