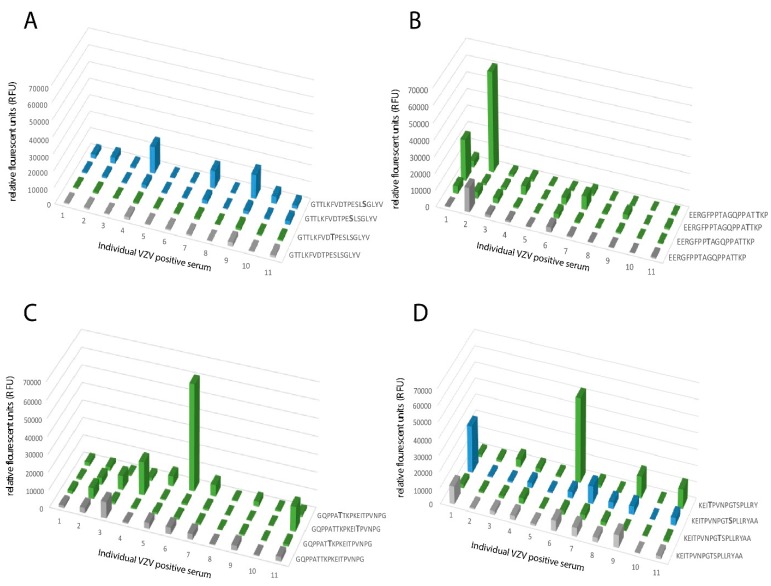
Figure 6.
A heterogenous response towards synthetic peptides, with single or multiple GalNAc modifications, was observed for peptides covering parts of gE close to the transmembrane domain, including the MLD domain when individual serum samples were analyzed in detail. (A) Synthetic peptide 460-GTTLKFVDTPESLSGLYV-477 unmodified or modified by GalNAc residues at either Ser or Thr. (B) Synthetic peptide 505-EERGFPPTAGQPPATTKP-522 unmodified or modified by GalNAc residues at Thr. (C) Synthetic peptide 514-GQPPATTKPKEITPVNPG-531 unmodified or modified by GalNAc residues at Thr residues. (D) Synthetic peptide 523-KEITPVNPGTSPLLRY-540 unmodified or modified by GalNAc at either Ser or Thr residues. Grey: unglycosylated peptide. Blue: GalNAc modification on a Ser residue. Green: GalNAc modification on a Thr residue. X-axis shows the individual serum samples (n = 11) from VZV IgG-positive patients, plotted in the same order of individual sera in all graphs.