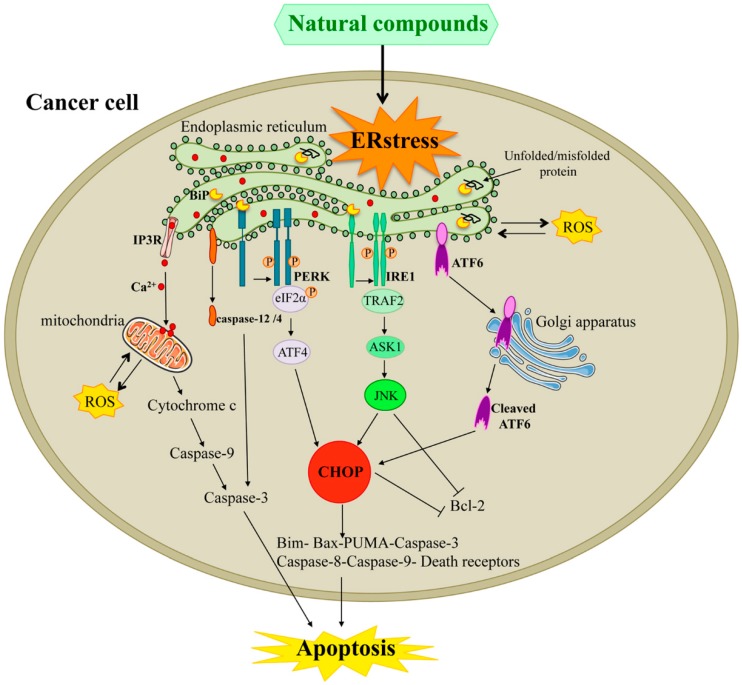
Figure 1.
ER stress-related apoptosis triggered by natural compounds. If the adaptive UPR pathway is not able to restore the ER function, upon severe or prolonged ER stress, activation of ER stress sensors can lead to apoptosis. A lot of natural compounds can induce ER stress, which leads to activation of the three ER sensors. Dissociation of BiP from all three sensors PERK, IRE1, and ATF6 leads to generation of their active forms. Active PERK dimerizes, autophosphorylates, and via the eIF2α/ATF4/CHOP pathway, modulates intrinsic and extrinsic apoptosis pathways. Active IRE1 has been demonstrated to induce the expression of Bcl-2 family members both via CHOP and via TRAF2/ASK1/JNK. Cleaved ATF6 can activate the induction of the pro-apoptotic transcription factor CHOP and consequently regulate Bcl-2 family members expression. All three branches of UPR can act concertedly to trigger both mitochondrial and death receptors apoptosis. Moreover, the Ca2+ release from ER can activate the ER-resident caspase-12/4, which in its active state, can promote the caspase-3 activation leading ultimately to apoptosis. Moreover, in ER stress conditions, oxidative stress induces the calcium leakage from ER and its subsequent uptake by the mitochondria leading to releasing cytochrome c from the mitochondrial matrix. Upon ER stress conditions, Ca2+ release from ER and mitochondrial ROS production alter cellular homeostasis and trigger apoptosis. Abbreviations used in Figure 1: ASK1: apoptosis signal-regulating kinase; ATF6: activating transcription factor 6; ATF4: activating transcription factor 4; Bax: (Bcl-2)-associated X protein; Bcl-2: B-cell lynphoma2; BiP: binding immunoglobulin protein; CHOP: C/EBP homologous protein; eIF2α: Eukaryotic initiation factor 2α; ER: endoplasmic reticulum; IP3R: inositol 1,4,5,-triphosphate receptor; TRAF2: tumor necrosis factor receptor-associated factor 2; JNK: JUN N-terminal kinase; ROS: reactive oxygen species.