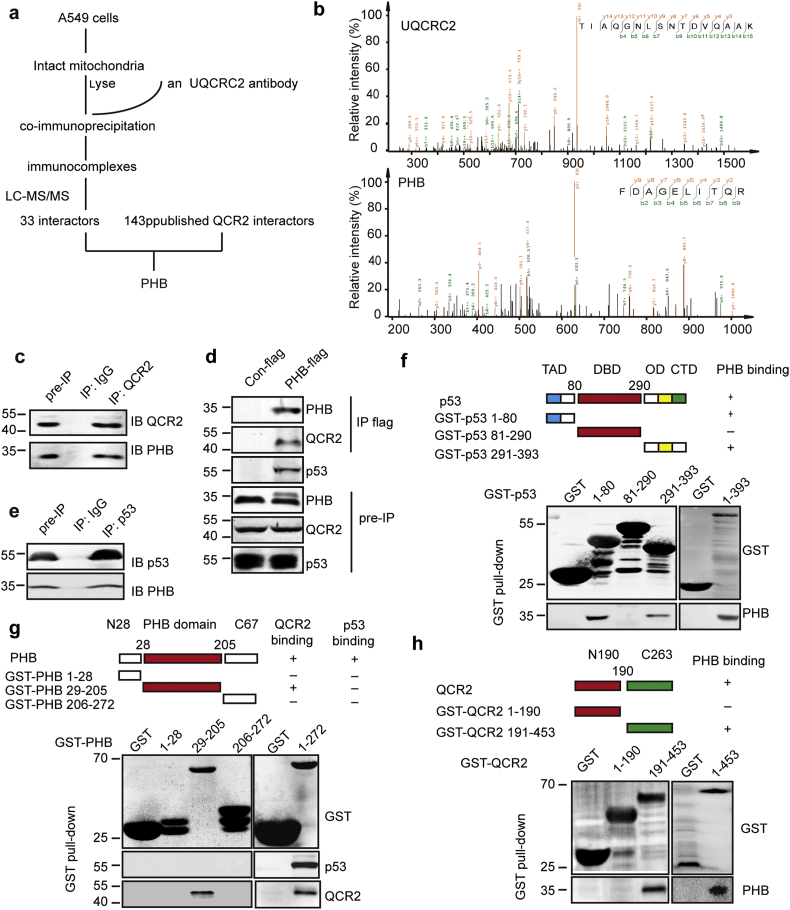
Fig. 4.
QCR2 interacts with PHB, a p53 chaperone. (a) Flowchart to identify proteins interacting with QCR2: intact mitochondria from A549 cells were isolated, and the mitochondrial lysates were used for co-immunoprecipitation studies using an anti-QCR2 antibody or control IgG. Immunocomplexes were analyzed by LC-MS/MS. Combined with the analysis with 143 published UQCRC2/QCR2 interactors, PHB was identified as a potential QCR2 target protein. (b) The spectra of QCR2 and PHB obtained by LC-MS/MS. (c) Intact mitochondria from A549 cells were isolated, and the mitochondrial lysates were used for co-immunoprecipitation studies using an anti-QCR2 antibody or control IgG. Immunocomplexes were analyzed by western blotting for QCR2 and PHB. (d) Plasmids encoding Con-flag or PHB-flag were transfected into HCT116 cells. Whole cell lysates were then used for co-immunoprecipitation studies using an anti-flag antibody. Cell lysates and immunocomplexes were analyzed using anti-QCR2, anti-p53 and anti-PHB antibodies. (e) HCT116 cells treated with PS-341 for 4 h were isolated, and the lysates were immunoprecipitated using an anti-p53 antibody or control IgG. Immunocomplexes were analyzed by western blotting for p53 and PHB. (f) Mapping the PHB binding domain on p53 by GST pull-down. Purified GST-p53 proteins were incubated with cell extracts prepared from 293 cells and then detected by western blotting for PHB. (g) Mapping the QCR2 binding domain on PHB by GST pull-down. Purified GST-PHB proteins were incubated with cell extracts prepared from 293 cells treated with PS-341 and detected by western blotting for QCR2 and p53. (h) Mapping the PHB binding domain on QCR2 by GST pull-down. Purified GST-QCR2 proteins were incubated with cell extracts prepared from 293 cells and then detected by western blotting for PHB.