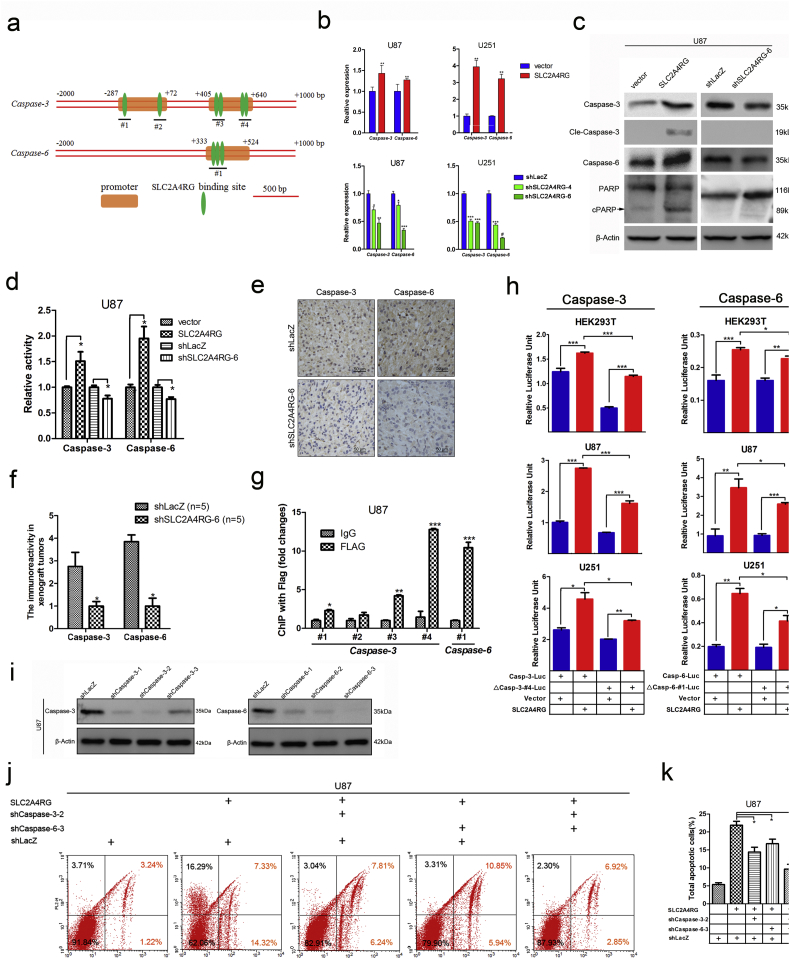
Fig. 5.
SLC2A4RG induces cellular apoptosis via direct transactivation of caspase-3 and caspase-6. (a) A schematic drawing of potential SLC2A4RG DNA binding sites in the promoter region for caspase-3 or caspase-6. (b) The mRNA expressions of caspase-3 and caspase-6 in U87 and U251 cells with overexpressed or depleted SLC2A4RG detected by RT-PCR. (c) Western blot confirming the protein levels of caspase-3, caspase-6, and PARP in SLC2A4RG-overexpressed or -silenced U87 cells. β-Actin serves as the loading control. (d) Detection of the relative enzymatic activity of caspase-3 and caspase-6 in U87 cells with overexpression or knockdown of SLC2A4RG. (e) IHC analysis of caspase-3 and caspase-6 in intracranial tumors developed from SLC2A4RG silenced or control U87 cells. (f) The expression scores of caspase-3 or caspase-6 in the two groups. (g) Exploration and validation of SLC2A4RG binding sites depicted in (a) with ChIP-PCR. (h) Dual-luciferase reporter assay determining the function of #4 site or #1 site on the expression of caspase-3 or caspase-6 when regulated by SLC2A4RG in HEK293T, U87, and U251 cells. (i) Western blot is analyzing the efficiency of shRNAs targeting caspase-3 or caspase-6 in U87 cells. β-Actin serves as the loading control. (j and k) Flow cytometry with Annexin V and 7-AAD staining determining the changes of SLC2A4RG-induced apoptosis in U87 cells after inhibiting caspase-3 or/and caspase-6. Results analyzed by t-test presented as mean ± SEM. ns, no significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.