Figure 3: Z1-Y15 alginate protects viable and glucose responsive allogeneic islets in non-human primates without any immunosuppression.
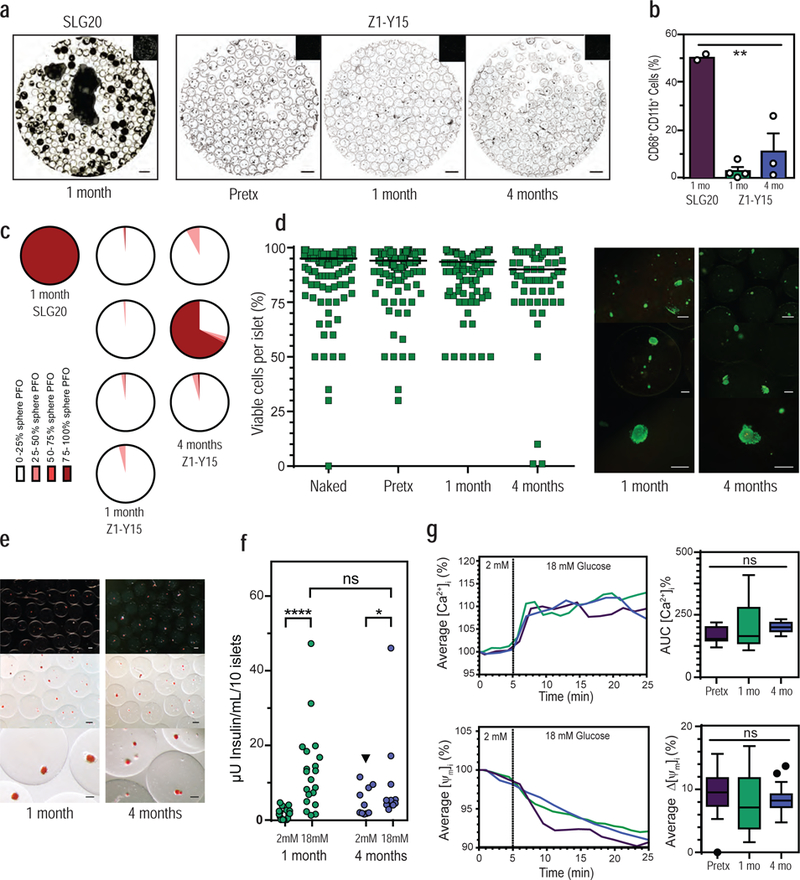
(a) Representative inverted phase contrast of retrieved spheres: SLG20 empty spheres at 1 month, Z1-Y15 spheres with encapsulated allogeneic islets pre-transplantation (pretx), post retrieval from NHP at 1 month, and 4 months. (b) Percentages of CD68+/CD11b+ macrophage populations dissociated from retrieved sphere surfaces using flow cytometry ( ** p = 0.0013; one-way analysis of variance (ANOVA) with bonferroni multiple-comparison correction; SLG20 n = 2 NHP; Z1-Y15 1 month n = 4 NHP; Z1-Y15 4 month n = 3 NHP; mean ± SEM; individual data points overlaid). (c) Histograms depict the degree of PFO sphere coverage for each of the primate retrievals at 1 month (SLG20 and Z1-Y15) and 4 months (Z1-Y15) post-transplantation in NHP. White and light colors depict little sphere coverage by fibrosis and dark red colors depict spheres mostly fibrosed. The SLG20 spheres with encapsulated islets were completely adhered to omental tissue and are depicted as mostly fibrosed dark red. (Z1-Y15 encapsulated islets at 1 month: 121, 90, 152, and 125 spheres assessed from n = 4 NHP; Z1-Y15 encapsulated islets at 4 months: 160, 167, and 128 spheres assessed from n = 3 NHP). (d) Estimated percentages of viable cells within islet cell clusters following live/dead staining performed pre-encapsulation (naked), post-encapsulation in Z1-Y15 alginate (pretx), and post retrieval of non-fibrosed spheres at 1 month and 4 months (naked: n = 144 total islets assessed, median viability 95.0%; post-encapsulation (pretx): n = 191 total islets, median viability 94.0%; Z1-Y15: 1 month retrieval: n = 133 total islets over 4 NHP retrievals, median viability 93.5%; Z1-Y15: 4 month retrieval: n = 69 total islets over 2 NHP, median viability 90.0%; 25–50 islets were scored per primate; median value per group depicted). Viability scores were analyzed using the Wilcoxon signed rank test to assess whether median viability was significantly different from 75%. Seventy-five percent was chosen as the biological reference because it is the cutoff for islet preparations suitable for clinical intraportal islet transplantation. Six out of seven encapsulated islet preparations demonstrated significantly higher viability than 75% from pre-encapsulation (naked), post-encapsulation (pretx), to post retrieval from NHP ( p < 0.05; Wilcoxon signed rank test; two tailed; individual p values presented in supplementary table 6). One primate retrieved at 4 month presented with fibrosis and non-viable islets. Representative fluorescent images of retrieved Z1-Y15 encapsulated allogeneic islets with live/dead stains fluorescein diacetate (FDA) and propidium iodide (PI) at 1 month and 4 months. (e) Retrieved Z1-Y15 encapsulated allogeneic islets at 1 month and 4 months stained with the red dye dithizone for macroscopic detection of mature insulin granules within engrafted endocrine tissue (representative of the n = 6/7 retrieved NHP). (f) Glucose stimulated insulin secretion of viable retrieved Z1-Y15 encapsulated allogeneic islets showing secreted insulin levels per 10 islets when incubated in a low glucose solution (2 mM glucose) for 1 hour and high glucose solution (18 mM glucose) for 1 hour. The Z1-Y15 spheres with encapsulated allogeneic islets samples secreted higher levels of insulin under high glucose compared to low glucose at 1 and 4 months retrievals. Insulin levels under low glucose were significantly higher when comparing 1 month and 4 month retrievals (black triangle p = 0.01), yet high glucose insulin levels were not significant when comparing 1 month and 4 months (p = 0.21). (**** p < 0.0001; * p = 0.03; Wilcoxon matched-pairs signed-rank test (comparing low to high at 1 month and 4 months); unpaired Mann-Whitney test (comparing lows or highs at 1 month and 4 months); both two-tailed; 1 month: n = 4–6 replicates of 10 islets per n = 4 independent NHP retrievals, n = 19 total samples; 4 month: n = 5 replicates of 10 islets per n = 2 independent NHP retrievals; n = 10 total samples, individual data points shown). (g) Average islet traces of intracellular calcium influx ([Ca2+]i) and mitochondrial potential changes (ΔΨm) of Z1-Y15 spheres with encapsulated allogeneic islets in response to a perifusion based glucose challenge (left column). Assessments were performed pre-transplantation, post 1 month retrieval, and post 4 month retrieval. Average AUC [Ca2+]i % of intracellular calcium influx during time of 18 mM glucose stimulus (5–25 min) (top right). Average percent decrease in mitochondrial potential changes (ΔΨm % change) after response to 18 mM glucose stimulus at 25 min (bottom right). ( ns p > 0.05; one-way analysis of variance (ANOVA); pre-transplantation: n = 31 individual islets assessed; 1-month retrieval: n = 17 individual islets; 4-month retrieval: n = 20 individual islets; box and whisker with median, upper and lower quartile ranges, outliers, and 1.5× IQR).
