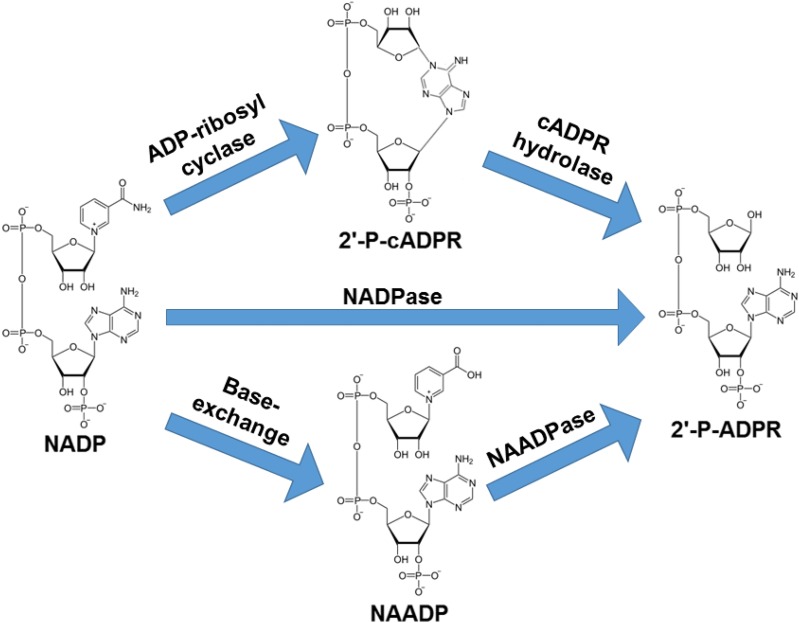
Fig. 1.
Enzymatic functions of CD38 with NADP as substrate. CD38 has been shown to primarily function as an NADPase (NADP hydrolase) converting NADP to 2′-P-ADPR. A small fraction of NADP is converted by CD38 to potent calcium signaling molecule 2′-P-cADPR as a part of its ADP-ribosyl cyclase function. The formed 2′-P-cADPR can then be hydrolyzed to 2′-P-ADPR (cADPR hydrolase). At acidic pH and in the presence of nicotinic acid, CD38 can convert NADP to another potent calcium-mobilizing second messenger, NAADP, in the base-exchange reaction. In this exchange, nicotinamide of NADP is replaced with nicotinic acid. Formed NAADP can be broken down to 2′-P-ADPR in an NAADP hydrolase reaction (NAADPase).