Figure 3.
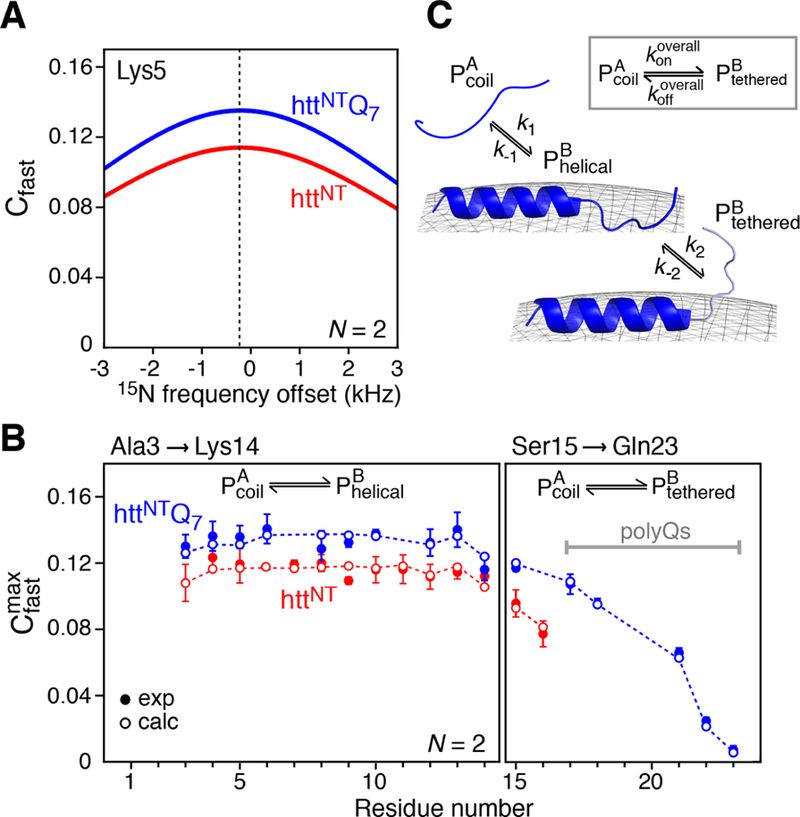
Simulated and experimental Cfast profiles for the binding of httNT and httNTQ7 to SUVs. (A) Simulated profiles of Cfast as a function of 15N frequency offset for Lys5 of httNT (red) and httNTQ7 (blue) in the presence of SUV vesicles at a molar ratio of 1:4 peptide to lipid. The curves were calculated using a variant of eq 7 that accounts for Δω ≠ 015) with the following values for the exchange parameters (where the first value refers to httNT and the second to httNTQ7): kex = 204.7/208.3 s−1, pB = 0.07/0.08, = 1.5/1.5 s− 1, = 2.4/3.6 s− 1, = 2300/2300 s−1, ω1 field strength = 2000 Hz, number of spin locks N = 2, and Δω/(2π) = −211.8 Hz (−3.47 ppm at 600 MHz). (B) Experimental 15N- profiles measured for httNT (red filled-in circles) and httNTQ7 (blue filled circles) in the presence of SUVs. The open circles are the best-fit profiles obtained by including into the target function together with the DEST and ΔR2 data (see Experimental Section). (C) Kinetic scheme used for modeling the binding of httNT and httNTQ7 to the surface of SUVs. The data for residues 3−14 are fit to a two-state exchange between the free peptide and membrane-bound helical peptide For residues 15−16 of httNT and 15−23 of httNTQ7, there is an additional process involving the interconversion between and , where the residues in the state are not attached to the membrane but tethered via the membrane-bound helical residues. The overall process for the conversion of to (via ) is shown in the inset.
