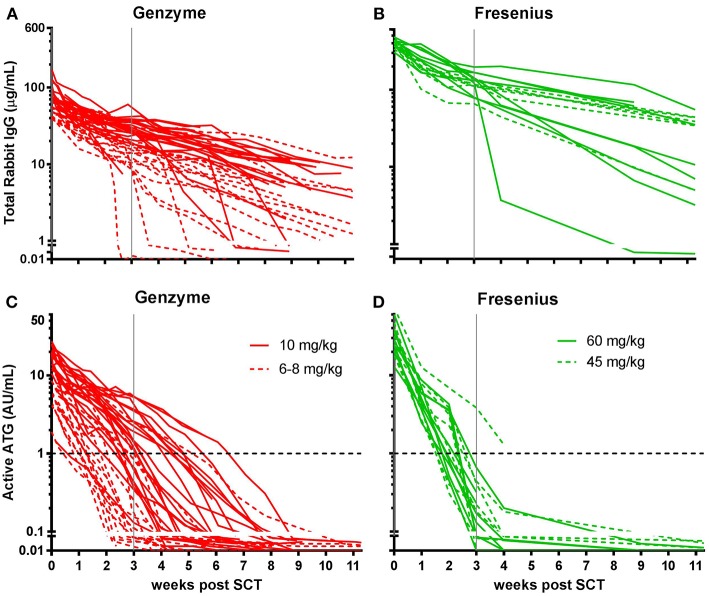
Figure 1.
Serum/plasma concentration of total Rabbit IgG (total ATG) and active ATG in ATG-GENZ (Genzyme, red) or ATG-FRES (Fresenius, green) treated patients. Total Rabbit IgG (μg/mL) of ATG-FRES treated patients (B) was higher in comparison with ATG-GENZ treated patients (A), because of the higher dose of ATG-FRES given, but clearance was comparable for both brands. Active ATG levels (AU/mL) of ATG-FRES treated patients (D), receiving 60 mg/kg (solid lines; n = 9) or 45 mg/kg (dashed lines; n = 7), decreased fast and was only in 1 out of 16 patients (6%) above 1 AU/mL at 3 weeks post-HSCT. ATG-GENZ treated patients (C) receiving 10 mg/kg (solid lines; n = 24) or 6–8 mg/kg (dashed lines; n = 18) showed more variance in clearance. At 3 weeks post-HSCT 15 out of 24 patients (63%) receiving 10 mg/kg had active ATG levels above 1 AU/mL vs. only 2 out of 18 patients (11%) receiving 6–8 mg/kg. The horizontal dashed line at 1 AU/mL marks the highest serum/plasma concentration at which T-cells can reappear in the blood. The vertical solid gray line is the expected time of neutrophil engraftment. Of note, in the 6 patients receiving ATG-GENZ and showing a steep decline of rabbit ATG IgG antibodies against rabbit IgG were detected.