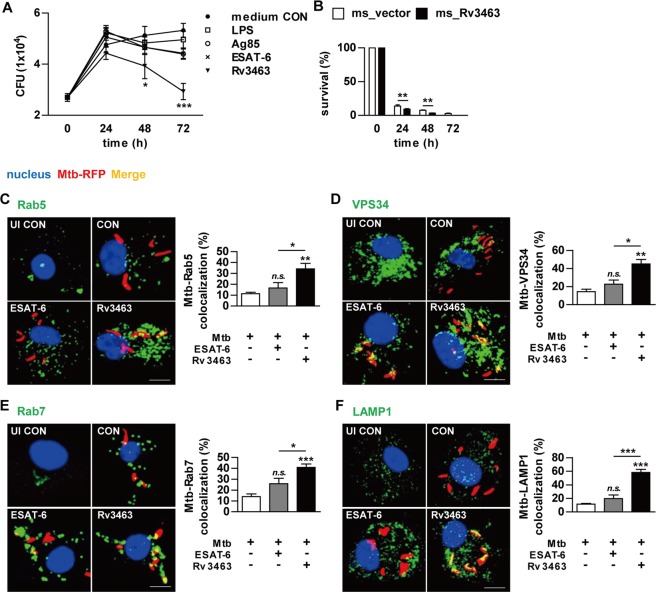
Figure 5.
Rv3463 inhibits the growth of intracellular Mtb through induction of phagolysosomal fusion in Mtb-infected macrophages. (A) Bone marrow-derived macrophages (BMDMs) were infected with Mtb at a multiplicity of infection (MOI) of 1 for 4 h, and then further treated with amikacin to kill extracellular bacteria for 2 h, washed three times, and incubated with or without 5 μg/ml Rv3463, 100 ng/ml LPS, 5 μg/ml Ag85, or 2 μg/ml ESAT-6 for 72 h. Intracellular Mtb growth was determined by plating the cell lysates on 7H10 agar for 0 to 72 h. (B) BMDMs were infected with M. smegmatis expressing Rv3463 (ms_Rv3463) and vector control strain (ms_vector) (MOI = 10) for 4 h, and then further treated with gentamycin for 2 h and washed three times. At 24, 48, and 72 h post-infection, intracellular bacterial growth was determined. Similar results were obtained for three independent experiments. **p < 0.01 for ms_Rv3463 compared to vector control strain. (C–F) BMDMs were infected with Mtb-RFP (MOI = 1) for 4 h, washed, incubated with or without 5 μg/ml Rv3463 or 2 μg/ml ESAT-6 for 72 h, fixed with 4% paraformaldehyde, and immunolabeled with anti-Rab5 (C), anti-VPS34 (D), anti-Rab7 (E), or anti-LAMP1 (F) antibodies, followed by Alexa 488-conjugated goat anti-rabbit IgG or anti-rat IgG (green). Cells were stained with DAPI to visualize the nuclei (blue). The localizations of the target molecules were analyzed by laser-scanning confocal microscopy. Scale bar, 10 μm. Bar graphs show the quantification of confocal data for the colocalization of Mtb and molecules detected. Values are mean ± SD of 50–100 cells per each experiment (n = 3). ***p < 0.001 for treatment compared to infection only controls (CON) or for difference between treatment data. n.s., no significant difference.