Table 1.
Optimization of reaction conditions a.
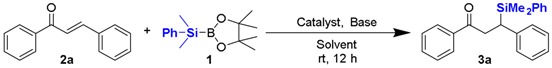 | ||||
| Entry | Catalyst | Base | Solvent | Yield (%) b |
| 1 | CS@CuSO4 | none | H2O | 30 |
| 2 | CS@Cu(OAc)2 | none | H2O | 22 |
| 3 | CS@Cu(acac)2 | none | H2O | 41 |
| 4 | CS@Cu(acac)2 | Na2CO3 | H2O | 50 |
| 5 | CS@Cu(acac)2 | K2CO3 | H2O | 65 |
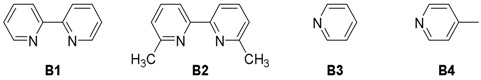
| 6 | CS@Cu(acac)2 | B1 | H2O | 40 |
| 7 | CS@Cu(acac)2 | B2 | H2O | 38 |
| 8 | CS@Cu(acac)2 | B3 | H2O | 86 |
| 9 | CS@Cu(acac)2 | B4 | H2O | 92 |
| 10 | - | B4 | H2O | NR |
| 11 | CS@Cu(acac)2 | B4 | DCM | NR |
| 12 | CS@Cu(acac)2 | B4 | THF | NR |
| 13 | CS@Cu(acac)2 | B4 | Et2O | NR |
| 14 | CS@Cu(acac)2 | B4 | Toluene | NR |
| 15 | CS@Cu(acac)2 | B4 | MeOH | 25 |
| 16 | CS@Cu(acac)2 | B4 | EtOH | 8 |
| 17 c | CS@Cu(acac)2 | B4 | H2O | 89 |
| 18 d | CS@Cu(acac)2 | B4 | H2O | 91 |
 | ||||
a Reaction conditions: substrate 2 (0.3 mmol), Me2PhSi-B(pin) 1 (1.2 equiv.), catalyst (5 mol % Cu loading), base (6 mol %), H2O (2 mL), room temperature, air, 12 h; b Isolated yield of product; c 2.5 mol % Cu loading was used; d Performed under Ar atomosphere.
