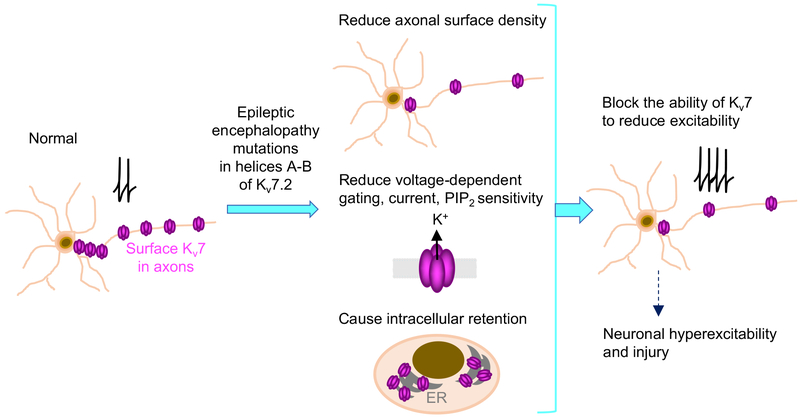
Figure 9. A working model for pathogenic mechanisms of epileptic encephalopathy mutations in helices A and B of Kv7.2.
Epileptic encephalopathy mutations (R333W, K526N, and R532W) localized peripheral to CaM contact sites in helices A and B reduced axonal surface expression of Kv7 channels, altered their sensitivity to PIP2, and disrupted their ability to inhibit excitability in hippocampal neurons. In contrast, M518V mutation at the CaM contact site in helix B severely decreased CaM binding, axonal surface expression, and voltage-dependent activation. Intracellular accumulation of Kv7.2-M518V also caused cell death. We propose that a combination of these multiple yet diverse defects in Kv7 channels could exert more severe impacts on neuronal excitability and health, and thus serve as pathogenic mechanisms underlying Kcnq2 epileptic encephalopathy.