Scheme 3.
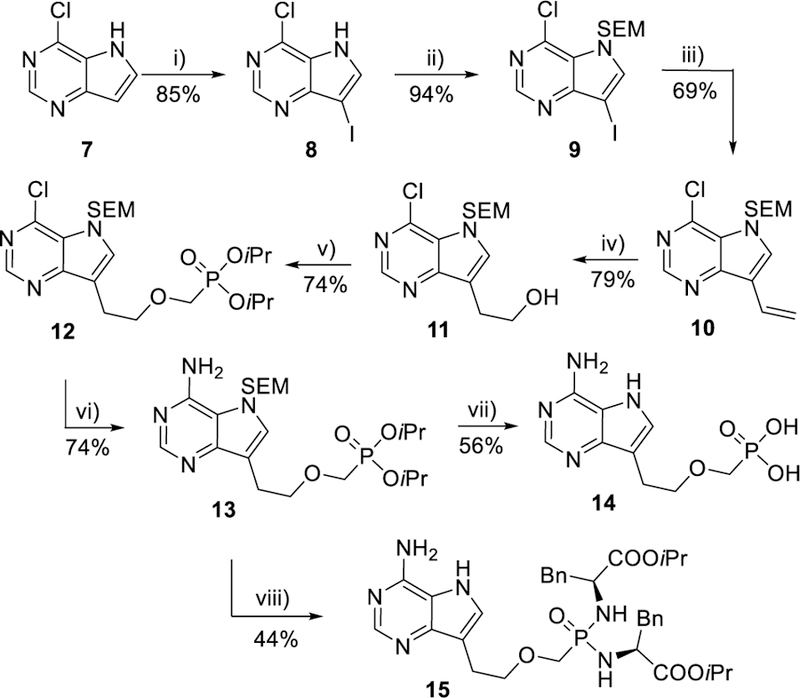
Synthesis of 9-deazapurine derivatives 14 and 15. Reagents and conditions: i) NIS, THF, rt; ii) (CH3)3Si(CH2)2OCH2Cl, NaH, DMF, rt; iii) CH2=CHSnBu3, Pd(t-Bu3P)2, THF, rt; iv) 9-BBN, THF, 0 °C to rt; then aq. NaBO3; v) n-BuLi, CF3SO2OCH2P(O)(OiPr)2, THF, −78 °C; vi) EtOH/NH3, 100 °C; vii) HCl 2 eq, H2O,130 °C; viii) TMSBr/TMSI, pyridine, rt; then (L)-NH2CH(Bn)COOiPr·HCl, PPh3, Aldrithiol-2, pyridine, Et3N, 70 °C.
