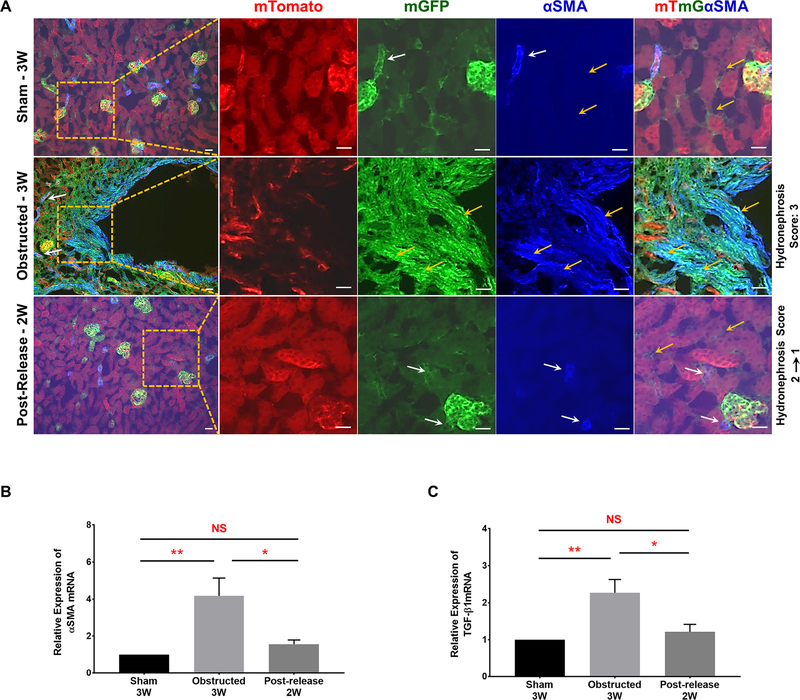
Figure 11. Release of obstruction reverses pericyte cell fate changes to α-SMA positive myofibroblasts:
(A) Presence of α-SMA was observed in renal arteries and arterioles. It co-localized with all the GFP+ Foxd1 lineage cells (white arrows) except the interstitial pericytes (yellow arrows) of sham-operated Foxd1Cre;mTmG kidneys. In 3W obstructed kidneys, in addition to its presence in arteries and arterioles (white arrows), a remarkable expansion of α-SMA was seen in the interstitium. Interstitial α-SMA co-localized with the expanded GFP+ interstitial pericytes in the obstructed kidneys (yellow arrows) indicating their putative fate change to myofibroblasts. In released kidneys, α-SMA was absent in the GFP+ interstitial pericytes (yellow arrows) and was restricted only to arteries and arterioles similar to shams (white arrows). Scale bars, 20μm. (B and C) qRT-PCR analyses showed a significant increase in α-SMA and TGF-β1 mRNA levels in 3W obstructed kidneys (n=6) compared to similar age group shams (n=4). In released kidneys (n=5) the expression levels of α-SMA and TGF-β1 was not significantly different from shams (*P<0.05; **P<0.01; NS-Non significant).