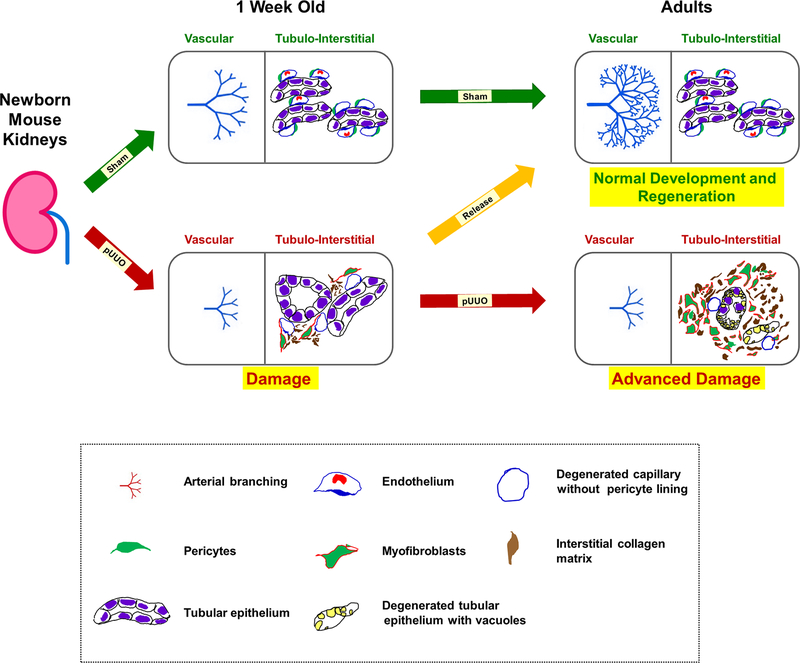
Figure 15. A proposed model of kidney injury and repair during obstruction and post-release in neonatal mouse kidneys:
Normal vasculogenesis and tubulogenesis through fetal and early postnatal life result in formation of the renal arterial tree and differentiated tubular epithelium in adulthood (green arrows). This process is dependent on a tightly regulated balance between a robust evolutionarily conserved morphogenetic program and constraints imposed by mutations, epigenetic factors, or environmental stressors, such as congenital urinary tract obstruction. Elevated levels of TGF-β1 mRNA observed during neonatal obstruction injury is proposed to increase the expression of α-SMA in pericytes, thereby transitioning them to collagen producing myofibroblasts. This cell fate change triggers a cascade of injury responses (red arrow) in the surrounding interstitium causing micro-vascular damage with pericyte effacement from the interstitial micro-vasculature and collagen deposition. Persistent obstruction advances the damage further (red arrow) due to continued transformation of pericytes to myofibroblasts. More importantly, the renal vasculature is severely affected with stunting of renal arterial branching resulting in decreased in RBF. In addition, there is tubular loss, migration of myofibroblasts to the interstitial space, and exacerbated collagen matrix deposition in the interstitium. Timely release of obstruction (orange arrow) impedes the tissue injury cascade by inhibiting the increases in TGF-β1 and α-SMA expression due to obstruction and reverses the balance to favor the normal pericyte phenotype, resumption of vascular branching, restoration of RBF and development of the nephron and collecting duct epithelium.