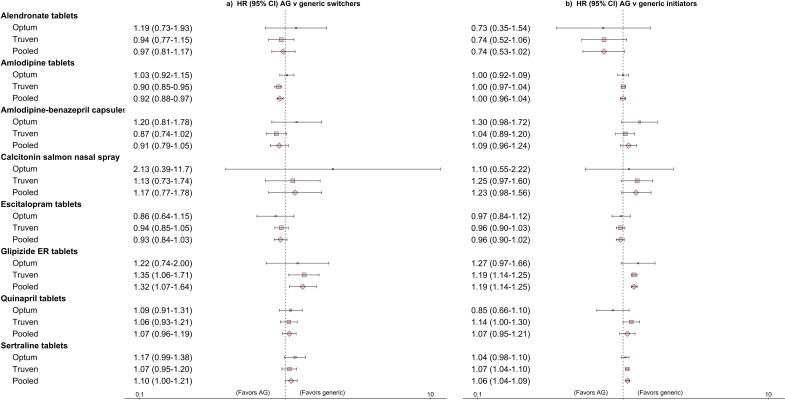
Fig 2. Hazard ratios (HRs) and 95% confidence intervals (CIs) comparing outcomes for patients initiating authorized generics (AGs) versus generics, and patients switching from brand-name products to AGs versus generics, after 1:1 propensity score matching in each database.
The outcome for amlodipine tablets, amlodipine-benazepril capsules, and quinapril tablets was a composite endpoint comprising hospitalization for myocardial infarction, ischemic stroke, or coronary revascularization procedures. The outcome for alendronate tablets and calcitonin salmon nasal spray was a composite non-vertebral fracture endpoint comprising humerus, wrist, hip, or pelvis fractures. The outcome for escitalopram tablets and sertraline tablets was hospitalization with a psychiatric condition as the principal discharge diagnosis code. The outcome for glipizide extended release (ER) tablets was initiation of insulin during the follow-up period.