Figure 1. (Graphical Abstract): Antigen receptor induced Ca2+ dynamics tune the patterns. of transcriptional activation.
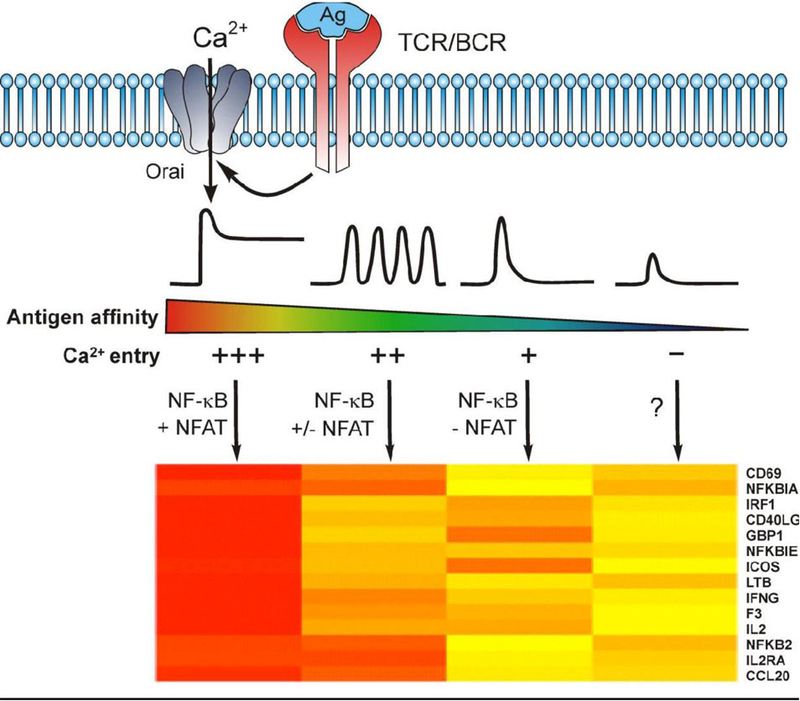
This schematic proposes how variations in antigen receptor induced Ca2+ dynamics elicit distinct patterns of gene expression depicted by a heatmap of differential gene expression. Work over the past 30 years has established that the strength of antigen receptor stimulation is encoded as quantitatively distinct patterns of Ca2+ signaling, and that these can each initiate a distinct transcriptionally driven fate of lymphocytes by activating Ca2+ dependent transcription factors. The key pro-inflammatory transcription factors NF-κB and NFAT, which exhibit differences in Ca2+ sensitive activation, decode differences in the strength of antigen stimulation into distinct patterns of transcriptional activation. High affinity antigen binding causes sustained STIM/Orai-dependent Ca2+ entry and these signals activate both NF-κB and NFAT. Intermediate antigen affinity/avidity interactions cause distinct patterns of signaling, possibly Ca2+ oscillations, that preferentially activate NF-κB and depending on the frequency can potentially activate NFAT. Low affinity/avidity interactions that cause a significant but transient elevation in cytoplasmic Ca2+ leading to the selective activation of NF-κB signaling and NF-κB dependent target gene expression. Finally, in the absence of Ca2+ entry, low amplitude, transient elevations in cytoplasmic Ca2+ may be insufficient for the activation of either NF-κB or NFAT.
