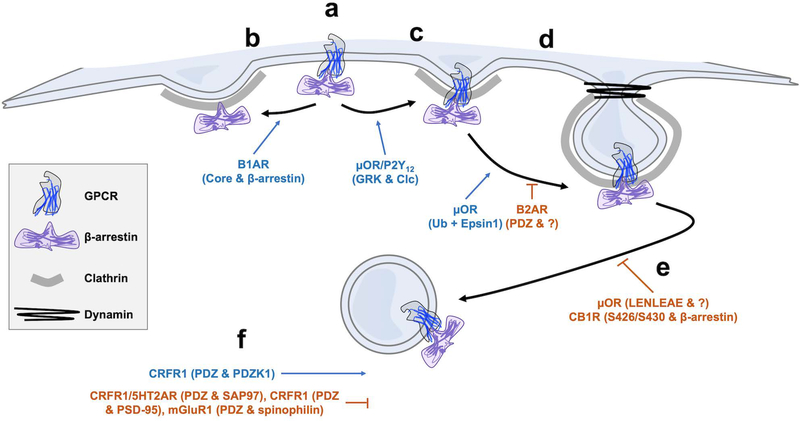
Figure 2:
GPCRs modulate endocytosis at distinct phases of the endocytic process. a) After ligand binding to a given receptor, β-arrestin is recruited to the receptor at the plasma membrane. b) In the case of B1AR, an interaction between β-arrestin and the B1AR core region causes β-arrestin to sort to clathrin-coated pits (CCPs) independent of the receptor. Other GPCRs sort with arrestin to CCPs. c) P2Y12 and μOR regulate clathrin light chain (CLC) phosphorylation through the activation of GPCR related kinases (GRKs) which is permissive of endocytosis continuing. d) After receptors are sorted into nascent CCPs, μOR is ‘proofread’ by Epsin1 to ensure that it is ubiquitinated before CCP maturation continues. At about the same phase, the PDZ ligand of B2AR delays recruitment of the GTPase dynamin through an unknown protein partner. e) After dynamin recruitment, μOR can delay dynamin-dependent scission through an unknown protein interacting with its C-terminal LENLEAE motif. CB1R, through an arrestin interaction mediated by two serines on its C-terminal tail, can also delay CCP lifetimes. f) Through as yet unknown mechanisms, GPCR interactions with PDZ domain containing proteins can globally upregulate (e.g. CRFR1 & PDZK1) or downregulate (e.g. mGluR1 & spinophilin) receptor internalization.