Abstract
Background:
The current upper-frequency cutoff of 150 Hz sometimes causes loss of pacemaker spike and misdiagnosis. We hypothesized that low-pass filter (LPF) other than 150 Hz could improve the detection of pacemaker spike. This study aimed to examine the effect of different LPF on pacemaker spike detection in remote and bedside electrocardiogram (ECG).
Methods:
Patients with permanent pacemaker implantation were included during routine follow-up. Standard 12-lead ECGs at 6 different upper-frequency cutoff (40, 100, 150, 200, 300, and 400 Hz) were collected. All ECGs were then transmitted to the remote clinic center. Ventricular and atrial pacing were analyzed by 2 independent medical practitioners.
Results:
A total of 88 patients’ ECGs were analyzed (mean age 73.8 ± 10.2 years and 85 with dual-chamber pacemakers). About 75.3% (64/85) of patients were diagnosed as atrial pacing by pacemaker programming. Among 6 different upper-frequency cutoff, the 300 Hz turned out to perform best in detecting atrial-paced spike (area under the curve [AUC] = 0.73, 95% confidence interval [CI]: 0.61–0.84 vs. 0.56, 95% CI: 0.61–0.84 at 150 Hz; P = 0.002) on bedside ECGs. Using programming as the golden standard, the 300 Hz LPF has a sensitivity of 59.4%, specificity of 85.7%, positive predictive value of 92.7% and negative predictive value of 40.9% on bedside ECGs. As for the ventricular pacing, the 300 Hz LPF also had a higher accuracy (AUC = 0.93; 95% CI = 0.84–1.00) than that at 150 Hz (AUC = 0.86; 95% CI: 0.77–0.94; P < 0.001) in detecting ventricular-paced spike on bedside ECGs. The results of remote ECGs were similar with bedside ECGs.
Conclusions:
A filter of 300 Hz cutoff may be recommended for ECG spike detection. With the recommended parameter, remote ECG can perform as well as bedside ECG.
Keywords: Digital electrocardiogram, Pacemaker spike, Upper-frequency cutoff, Remote electrocardiogram
Introduction
Electrocardiogram (ECG) is widely used by routine clinical practices to better understand the working of the heart in all its details, and the cause of a large variety of cardiovascular disease, since it was invented by Willem Einthoven in 1902. Recently, remote ECG diagnostic system developed rapidly and achieved more and more attention with the development of information and communication techniques. But the problem is that interpretations by several cardiologists reading the same ECG often vary substantially, especially for the remote ECG of pacemaker.[1–3] And there is still no evidence-based minimum number of ECG interpretations that is ideal for attaining or maintaining competency in ECG interpretation skills.[4,5] As we know, ECG is a primary evaluating tool for the patients with pacemaker implantation during follow-up, whether bedside or remote.[6,7] Different from general ECG, 1 key feature of pacemaker ECG is the pacemaker spike, which is a small signal just before the P wave or QRS wave. The identification of pacemaker spike is important for the accurate diagnosis of pacemaker ECG. However, the small spike signal is becoming more and more difficult to discern with the widely use of dipolar pacemakers.[8–10] Furthermore, some ECG information such as pacemaker spike signal may become weaker and even get lost during the remote transmission.[6,11] Consequently, many ECGs of pacemaker are misdiagnosed.[12] Therefore, it is urgent to improve the detection of pacemaker spike of remote and bedside ECG.
The signal intensity of pacemaker spike is related with many factors including signal processing of ECG device. One typical filter process is the anti-aliasing and upper-frequency cutoff (also called low-pass filter, LPF).[8] The upper-frequency cutoff of 150 Hz, recommended by the AHA/American College of Cardiology (ACC) guidelines today, always results in loss of spike.[7,13] we hypothesized that LPF other than 150 Hz could improve the detection of pacemaker spike of remote and bedside ECG. Therefore, in the present study, we focused on the pacemaker spike detection at different LPF and compared the diagnostic accuracy of remote and bedside ECG.
Methods
Ethical approval
This study was approved by the Ethics Committee of the Hospital. Given its retrospective nature and the fact that data analysis was performed anonymously, it was exempt from obtaining informed consent from patients.
Study population
Of all the 109 consecutive patients with pacemaker implantation for routine follow-up at outpatient clinic from January 1, 2015 to June 30, 2015 in our hospital, 88 patients were included in this study, who met the following inclusion criteria of this study: (1) without atrial fibrillation; (2) without serious interference of limb shaking, such as in condition of Parkinson disease. Patients’ history was collected using patients’ chart reviews.
The pacemaker and ICD devices used in the study were from a variety of manufacturers including Medtronic (Minneapolis, MN, USA) and St. Jude Medical (St. Paul, MN, USA). The parameter set-up of the pacemakers was: output amplitude 2.0 to 3.5 V, pulse width 0.4 ms, bipolar.
ECG collection and recording
Standard 12-lead ECGs were recorded in the resting supine position using recommended standardized procedures the same time as the pacemaker programming. For each patient, bedside ECGs were collected at 6 different upper-frequency cutoff (40, 100, 150, 200, 300, and 400 Hz). All these ECGs were then transmitted to the remote clinical center. ECGs were recorded and stored at a sampling rate of 500 Hz and printed using a paper speed of 25 mm/s. The lower-frequency cutoff was 0.5 Hz.
The ECG device utilized was designed by MedEx Beijing Company (MCA-22-12UP). The system analyzes and stores ECGs based on the standard 12-lead hookup at a sensitivity of 10 mm/mV, an input impedance of more than 5 MΩ and an input electricity of <0.1 μA. The ECG acquisition was processed a noise level <15 μV and common-mode rejection more than 100 dB. Direct-current polarization voltage was ±300 mV and time constant more than 3.2 s with a proper damping. Figure 1 shows the data acquisition, processing, and storage diagram of equipment.
Figure 1.
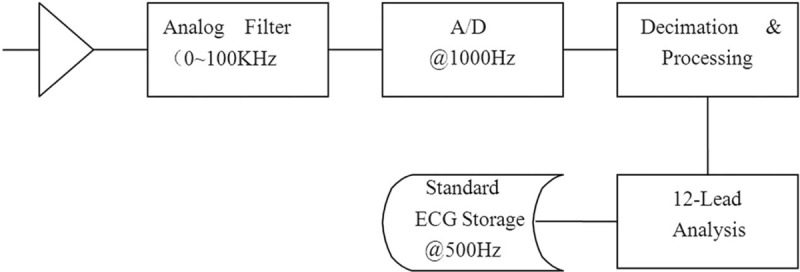
Data acquisition, processing and storage diagram of MCA-22-12UP used in this study.
ECG analysis
All ECGs were analyzed by 2 separate medical practitioners who had received training in pacer ECG reading and were unaware of patients’ treatment allocation or pacemaker programming outcome. The ECG was defined as “ventricular paced” if ventricular pacemaker spike can be recognized in more than 5 leads by both doctors. Atrial paced was defined as atrial spike can be seen in lead II and AVR. Baseline interference was defined as the absolute value of the baseline clutter more than 0.01 mV on at least 6 leads. In the absence of concordance between the 2 readers, a third (cardiologist) reporter adjudicated.
Statistical analysis
The Statistical Package for the Social Sciences for Windows (version 18.0; SPSS Inc., Chicago, IL, USA) was used for analysis. All continuous data are presented as mean ± standard deviation (SD) and categorical variables as count and percent and compared by using Pearson Chi-squared or Fisher exact test. By using the programming result as the golden standard, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of different upper-frequency cutoff for the detection of pace spike were calculated. Receiver operating characteristic (ROC) curves were used in evaluating detection for spike at different LPF. Area under the curve (AUC) was also calculated. Agreement between bedside ECG and remote ECG was evaluated using Cohen kappa. A large kappa value implies stronger agreement, with κ = 1 being the perfect agreement. A good level of agreement was defined as κ ≥ 0.61.[14] All tests were 2-sided, and P value < 0.05 was considered to indicate statistical significance.
Results
Characteristics of the study subjects
The ECGs from a total of 109 patients with pacemaker implantation were collected. Twenty-one records were excluded from this study due to technical and other issues. Therefore, a total of 88 patients’ ECGs were analyzed. The mean age was 73.8 ± 10.2 years and 43 (48.9%) of them were females.
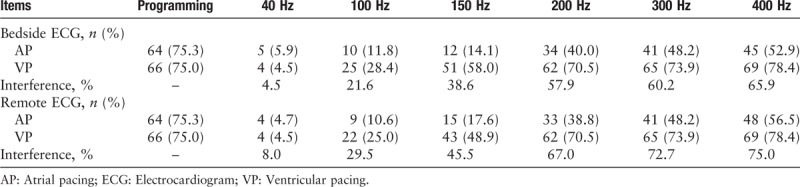
Among the 88 patients, 39 (44.3%) had sick sinus syndrome, 35(39.8%) had atrial-ventricular block, 11(12.5%) had tachycardia-bradycardia syndrome, and 3 (3.4%) had others. All patients’ pacemakers were bipolar leads and 85 (96.6%) were dual-chamber pacemakers. The results of pacemaker programming and ECG spike detection are summarized in Table 1. The interference rate of bedside and remote ECGs increased with upper-frequency cutoff, ranging from 4.1% to 65.9% and 8.0% to 75.0%, respectively.
Table 1.
Detection for ECG pacer spike at different low-pass filter.
Detection of atrial-paced spike
The performance of spike detection with different LPF is shown in Figures 2 and 3. As seen in Figure 2, the atrial-paced spike on bedside ECG can be found in lead V3 to V6 with an LPF of 40 Hz; in lead II, V1 to V6 with 100 Hz; lead I, II, avF, avR, and V1 to V6 with 150 and 200 Hz; and lead I, II, III, avF, avR, and V1 to V6 with 300 Hz. Figure 3 shows the performance of remote ECG in atrial-paced spike detection with different LPF. The atrial-paced spike can be recognized in lead V2 and V4 with an LPF of 40 Hz; in lead II, V2 to V6 with 100 and 150 Hz; lead II, avR, and V2 to V6 with 200 Hz, and lead I, II, avF, avR, and V1 to V6 with 300 Hz.
Figure 2.
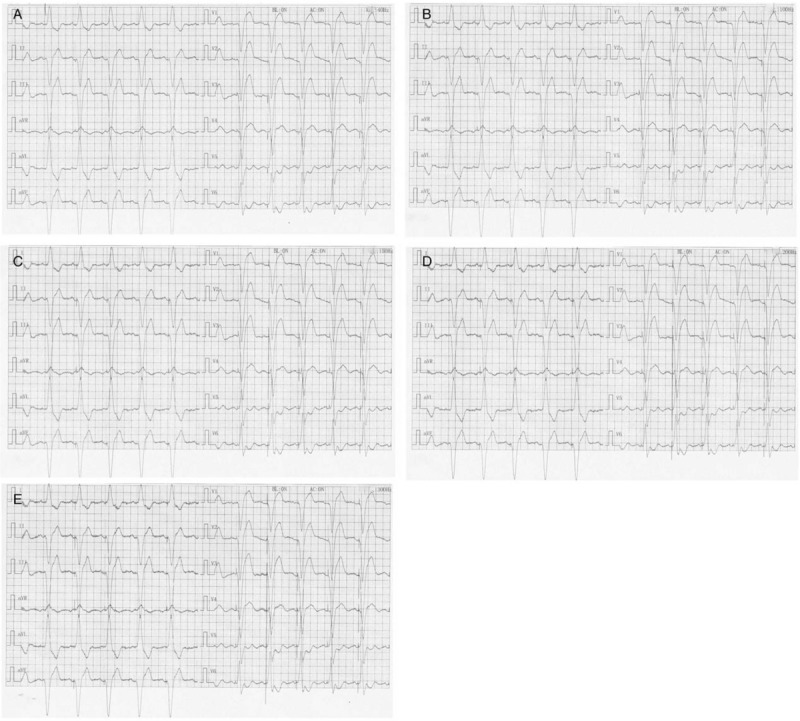
Detection of pacer spike on bedside electrocardiogram at different low-pass filter. (A) 40 Hz; (B) 100 Hz; (C): 150 Hz; (D) 200 Hz; (E) 300 Hz.
Figure 3.
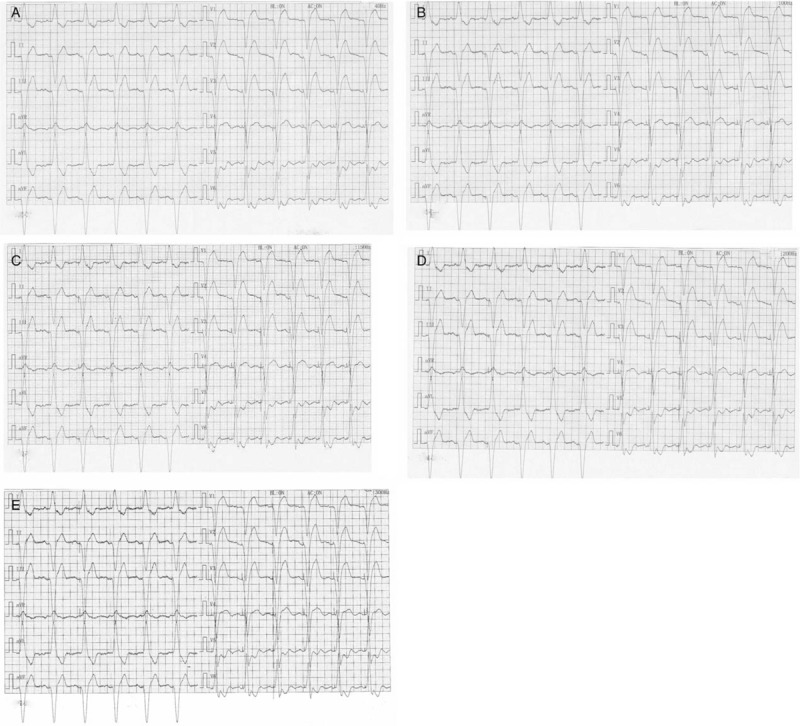
Detection of pacer spike on remote electrocardiogram at different low-pass filter. (A) 40 Hz; (B) 100 Hz; (C) 150 Hz; (D) 200 Hz; (E) 300 Hz.
Further analysis in detection of atrial spike presented notable difference among different upper-frequency cutoff [Tables 2 and 3]. A total of 85 atrial-paced patients were analyzed, with 64 of them were actually atrial paced while programming. By using the programming result as the golden standard, results of bedside ECG showed that the general 150 Hz LPF has a sensitivity of 17.2% and specificity of 95.2% on bedside ECGs. The PPV was 91.7% and NPV was 27.4%. With the increase of LPF, the recognition rate of atrial spike (showed as sensitivity) got higher, increasing from 7.8% to 62.5%. The PPV of all the 6 different LPF were high, ranging from 88.9% to 100%. The NPV was relatively low, which was 26.2% under 40 Hz and raised to 40% under 400 Hz. Moreover, the Cohen test showed that remote ECGs could perform as well as bedside ECGs (κ = 0.65–0.86), especially under an LPF of 300 Hz (κ = 0.86).
Table 2.
Results and diagnostic accuracy of different low-pass filter for detecting atrial-paced spike.
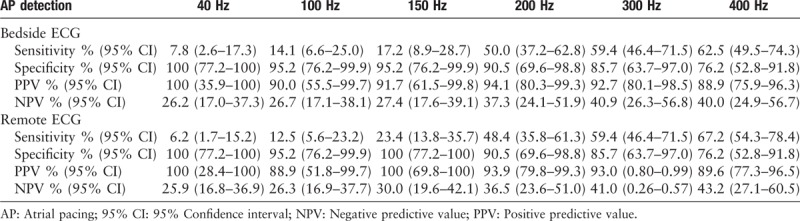
Table 3.
ROC curve for different left-pass filter in detecting atrial-paced spike and coherence between bedside and remote ECG.
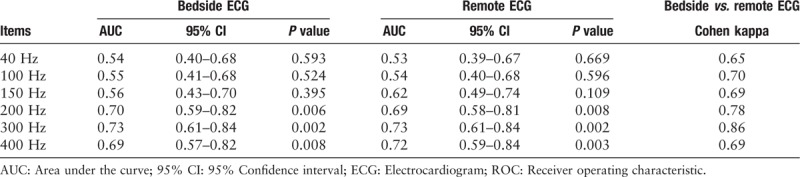
With regard to the ROC curve, the 300 Hz turned out to perform best in detecting atrial-paced spike compared with other filters. The requested 150 Hz had an AUC of 0.56 (95% CI: 0.43–0.70; P = 0.395) and a 300 Hz filter had an AUC of 0.73 (95% CI: 0.61–0.84; P = 0.002). By using the programming result as the golden standard, the ROC curves of bedside ECG [Figure 4A] and remote ECG [Figure 4B] were drawn.
Figure 4.
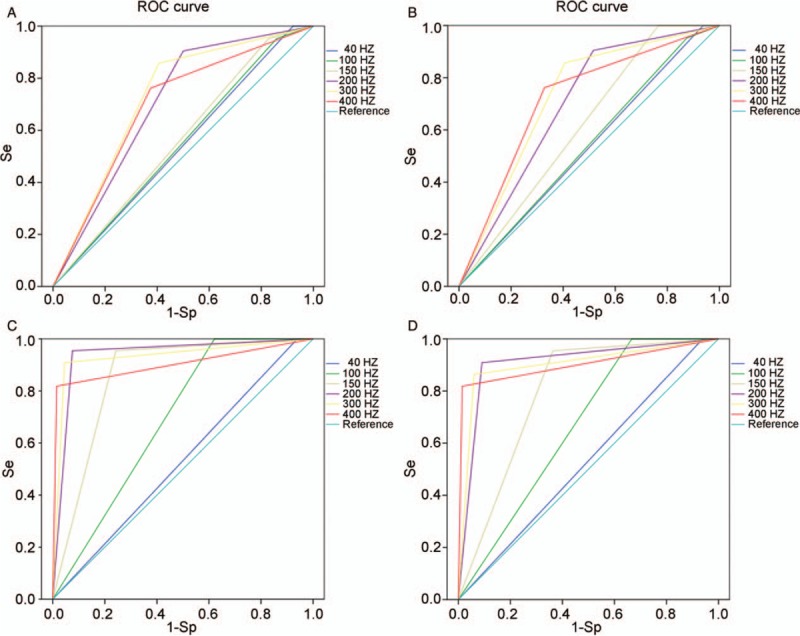
Receiver operating characteristic curve for pacer spike detection: (A) for bedside electrocardiogram (ECG) and (B) for remote ECG in atrial-paced spike detecting; (C) for bedside ECG and (D) for remote ECG in ventricular-paced spike detecting. Se: sensitivity; 1-Sp: 1-specificity.
Detection of ventricular pacemaker spike
Tables 4 and 5 displayed results of ventricular spike detection with both bedside and remote ECGs. Among the 88 ventricular-paced patients, programming results showed that 66 of them were actually ventricular paced. Generally, the detection rate of ventricular spike was higher than atrial spike. When LPF was 200 Hz or more, almost all the ventricular pacing signals could be identified. The sensitivity of 200, 300, and 400 Hz were 92.4%, 95.5%, and 98.5%, respectively. Similar with results of atrial spike detection, the PPVs were ranging from 94.2% to 100%. However, different LPF demonstrated quite different NPV, with 26.2% under 40 Hz and 94.7% under 400 Hz. The Cohen kappa test showed that the coherence between remote ECG and bedside ECG was low at a filter of 150 or 100 Hz and high at other filters.
Table 4.
Results and diagnostic accuracy of different left-pass filter for detecting ventricular-paced spike.
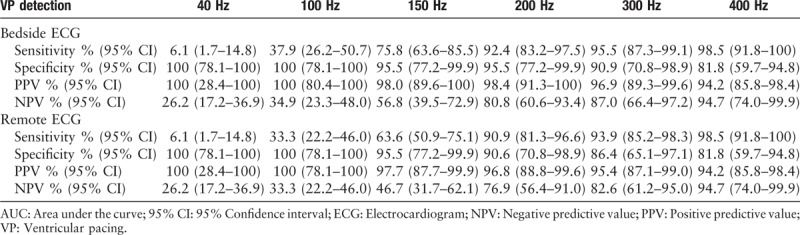
Table 5.
ROC curve for different left-pass filter in detecting ventricular-paced spike and coherence between bedside and remote ECG.
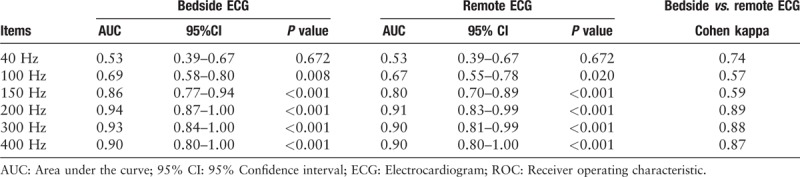
The ROC curve was also made based on the programming results [Figure 4C and 4D]. A filter of 300 Hz cutoff also had a higher accuracy (AUC = 0.93; 95%CI: 0.84–1.00; P < 0.001) than that at 150 Hz (AUC = 0.86; 95%CI: 0.77–0.94; P < 0.001) in detecting ventricular-paced spike on bedside ECGs. Similar results were observed on remote ECGs.
Discussion
We demonstrated that the modern ECG device with a recommended 150 Hz upper-frequency cutoff cannot reliably meet clinical requirements of pacemaker spike detection. Our results suggested that a filter of 300 Hz cutoff may be recommended for ECG spike detection. Moreover, the performance of remote pace ECG is in accordance with bedside ECG in condition of upper-frequency cutoff at 300 Hz.
With an increasing need for patients treated by pacemaker and their evaluating in outpatient clinic follow-up, the identification of pacemaker spike is attracting more and more attention.[2,15] Pacer stimuli signal, also called pacer signal or pacemaker spike, is a characteristic performance in a pacemaker ECG. It presents as a current deflection wave with short duration and large amplitude. The amplitude of a pacemaker spike varies with several causes including the processes of ECG device. One major parameter is the upper-frequency cutoff of ECG device. An upper-frequency cutoff, known as LPF, means that the filter passes low-frequency signals and has a cutoff frequency at this set parameter.[8]
A proper LPF is in urgent need to improve the ECG diagnosing. Clinicians always use a 40 Hz filter to make the electrocardiograph waves clear. However, with a filter of 40 Hz cutoff, the stimuli signal of pacemaker can be easily missed and the ECG may be diagnosed as left bundle branch block, atrial-ventricular node escape rhythm or ventricular escape rhythm.[8,10,13] Our research showed that only 7.8% atrial spike and 6.1% ventricular pacing signal can be recognized at this filter. Currently, the AHA/ACC/HRS guidelines today request a pass band up to 150 Hz for both adult and pediatric ECGs.[13,16] Extensive publications suggest the cutoff should be raised to 250 Hz at least for children. Moreover, ECG with a 150-Hz filter could miss detecting pacemaker stimuli.[7,11,17] In our study, a low rate of pacemaker spike identification at the fixed 150 Hz was observed (17.2% in atrial spike detection and 75.8% in ventricular spike). We found that more atrial stimuli pulse could be identified under the general sampling rate of 500 Hz when LPF was raised more than 150 Hz. However, it should be noticed that interference rate also increased and an appropriate filter should be provided. In this study, we analyzed 88 patients’ ECGs and exclaimed that a filter of 300 Hz cutoff would be a better choice for pacing ECGs, especially for atrial-paced ECGs.
The performance of ECG spike detection has been questioned since the widely use of bipolar leads in 1990s. It is known that amplitude of stimuli signal is related with the interval between the positive pole and negative pole. Technically, the positive pole of bipolar leads is located on the annular electrode and negative pole on the top of pacing electrode. This result in the shorter distance between 2 poles compared with unipolar leads, which is associated with a smaller pacer signal on the ECG. With a sampling rate of 500 to 1000 Hz, current signal processing system cannot check for bipolar stimuli pulse effectively and sampling standard should be updated to fit the current clinical requirements, especially for atrial stimuli signal. Clinician can adjust LPF appropriately to improve ECG performance in pacemaker spike diagnose and a 300-Hz filter may be a desirable choice.
With the development of website technique, remote ECG diagnostic system is popular and several remote ECG diagnostic platforms have been established in some district of Shanghai. Concerns have been raised about whether remote ECG system is suitable for spike detection. It has been proved that remote ECGs can perform well in the transmission and diagnosis of nonpaced patients. However, comprehensive study about diagnosis of remote pacemaker ECGs is still limited. In this study, the result indicated that, under a proper LPF, spike detection of remote ECGs performs as well as bedside ECGs.
We found that remote ECG had a higher recognition rate under higher LPF, but this may increase the interference rate. Our study showed that the baseline interference rate of remote ECGs was generally higher than bedside ECGs, which may result in false recognition. Thus, promotion of transmission system of remote ECG is still needed.
This study had an inherent major limitation of being a single-center research and the study population is small. We did some preliminary clinical studies in this field and discussed the results. Description of a stimuli pulse includes duration, amplitude, morphology, and direction. Many factors can also influence its performance. Here we only analyzed the impact of ECG device on amplitude of pacer signal. Other relative parameter should also be discussed further.
In conclusion, to avoid the loss of spike, we suggested that a new ECG standard should be developed and a filter of 300 Hz cutoff may be recommended. Under the recommended upper-frequency cutoff, the remote ECG can be performed as well as bedside ECG.
Funding
This work was supported by the project of Shanghai Municipal Education Commission (No. 11CXY39 to Yi-Gang Li), the State Key Program of National Natural Science Foundation of China (No. 81530015 to Yi-Gang Li), Shanghai Municipal Commission of Health and Family Planning (No. 20134119 to Jian Sun), Funding for Clinical Trial of Xinhua Hospital (No. 15LC15 to Jian Sun), and Shanghai Jiao Tong University School of Medicine grant (No. 13XJ10038 to Jian Sun).
Conflicts of interest
None.
Footnotes
How to cite this article: Sun J, Lu QF, Zhao Y, Zhang PP, Wang J, Wang QS, Liu XH, Li YG. A low-pass filter of 300 Hz improved the detection of pacemaker spike on remote and bedside electrocardiogram. Chin Med J 2019;132. doi: 10.1097/CM9.0000000000000110
References
- 1.Jeroudi OM, Christakopoulos G, Christopoulos G, Kotsia A, Kypreos MA, Rangan BV, et al. Accuracy of remote electrocardiogram interpretation with the use of Google Glass technology. Am J Cardiol 2015; 115:374–377. doi: 10.1016/j.amjcard.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Andersson HB, Hansen MB, Thorsberger M, Biering-Sorensen T, Nielsen JB, Graff C, et al. Diagnostic accuracy of pace spikes in the electrocardiogram to diagnose paced rhythm. J Electrocardiol 2015; 48:834–839. doi: 10.1016/j.jelectrocard.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Mulpuru SK, Madhavan M, McLeod CJ, Cha YM, Friedman PA. Cardiac pacemakers: function, troubleshooting, and management: part 1 of a 2-part series. J Am Coll Cardiol 2017; 69:189–210. doi: 10.1016/j.jacc.2016.10.061. [DOI] [PubMed] [Google Scholar]
- 4.Israel CW, Ekosso-Ejangue L, Sheta MK. Analysis of pacemaker ECGs [in German]. Herzschrittmacherther Elektrophysiol 2015; 26:260–273. doi: 10.1007/s00399-015-0383-5. [DOI] [PubMed] [Google Scholar]
- 5.Salerno SM, Alguire PC, Waxman HS. Competency in interpretation of 12-lead electrocardiograms: a summary and appraisal of published evidence. Ann Intern Med 2003; 138:751–760. [DOI] [PubMed] [Google Scholar]
- 6.Diemberger I, Gardini B, Martignani C, Ziacchi M, Corzani A, Biffi M, et al. Holter ECG for pacemaker/defibrillator carriers: what is its role in the era of remote monitoring? Heart 2015; 101:1272–1278. doi: 10.1136/heartjnl-2015-307614. [DOI] [PubMed] [Google Scholar]
- 7.Kligfield P, Gettes LS, Bailey JJ, Childers R, Deal BJ, Hancock EW, et al. Recommendations for the standardization and interpretation of the electrocardiogram: part I: The electrocardiogram and its technology: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2007; 115:1306–1324. doi: 10.1161/circulationaha.106.180200. [DOI] [PubMed] [Google Scholar]
- 8.Luo S, Johnston P. A review of electrocardiogram filtering. J Electrocardiol 2010; 43:486–496. doi: 10.1016/j.jelectrocard.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Luo S, Johnston P, Macfarlane PW. Implanted cardiac pacemaker pulses as recorded from the body surface. J Electrocardiol 2012; 45:663–669. doi: 10.1016/j.jelectrocard.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Rojas-Gonzalez A, Cecconi A, Merino JL, Jimenez-Borreguero LJ, Alfonso F. Spike or not a spike? That is the question in a patient with single lead pacemaker. J Electrocardiol 2017; 50:937–938. doi: 10.1016/j.jelectrocard.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Shurrab M, Pagacz T, Shauer A, Lashevsky I, Newman D, Crystal E. Pacing spikes all over. Clin Med Insights Cardiol 2017; 11:1179546817714478.doi: 10.1177/1179546817714478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Littmann L, Farrell RM. Potential misinterpretations related to artificial pacemaker signals generated by electrocardiographs. J Electrocardiol 2015; 48:717–720. doi: 10.1016/j.jelectrocard.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Bailey JJ, Berson AS, Garson A, Jr, Horan LG, Macfarlane PW, Mortara DW, et al. Recommendations for standardization and specifications in automated electrocardiography: bandwidth and digital signal processing. A report for health professionals by an ad hoc writing group of the Committee on Electrocardiography and Cardiac Electrophysiology of the Council on Clinical Cardiology, American Heart Association. Circulation 1990; 81:730–739. doi: 10.1161/01.CIR.81.2.730. [DOI] [PubMed] [Google Scholar]
- 14.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174. doi: 10.2307/2529310. [PubMed] [Google Scholar]
- 15.Venkatachalam KL. Common pitfalls in interpreting pacemaker electrocardiograms in the emergency department. J Electrocardiol 2011; 44:616–621. doi: 10.1016/j.jelectrocard.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Pettersson J, Carro E, Edenbrandt L, Maynard C, Pahlm O, Ringborn M, et al. Spatial, individual, and temporal variation of the high-frequency QRS amplitudes in the 12 standard electrocardiographic leads. Am Heart J 2000; 139 (Pt 1):352–358. doi: 10.1067/mhj.2000.101782. [DOI] [PubMed] [Google Scholar]
- 17.Rijnbeek PR, Kors JA, Witsenburg M. Minimum bandwidth requirements for recording of pediatric electrocardiograms. Circulation 2001; 104:3087–3090. doi: 10.1161/hc5001.101063. [DOI] [PubMed] [Google Scholar]


