FIGURE 3.
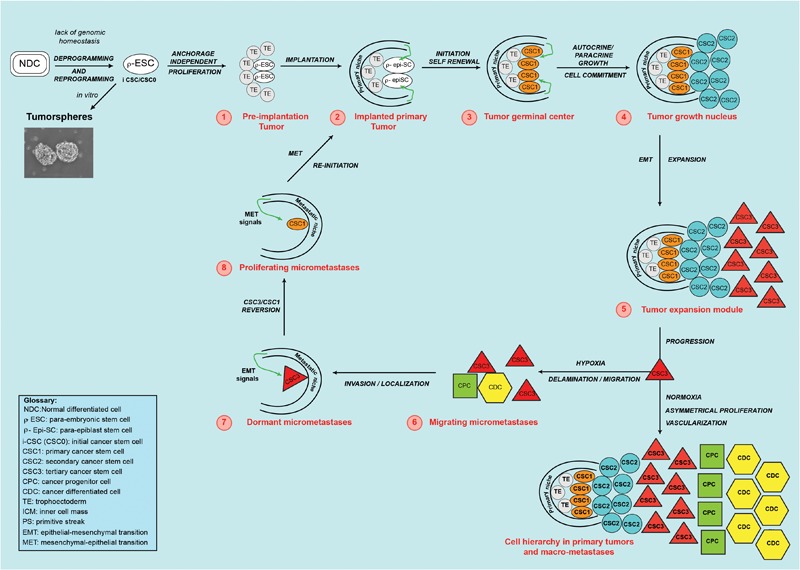
Theoretical cancer process. A normal differentiated cell (NDC), in the absence of genomic homeostasis, would be de-programmed and re-programmed to a para-ESC (p-ESC), constituting the initial cancer stem cell (i-CSC or CSC0). (1) An i-CSC, by anchorage-independent proliferation, would generate p-ESC progeny, forming an initial tumor in vivo (1-red) (or a tumorsphere in vitro), corresponding to a pre-implantation blastocyst (TE/ICM). (2) The initial tumor would install in a niche with proper factors and develop a primary tumor, mimicking an implanted blastocyst in the endometrium. (3) By PGRN activity, primary undifferentiated, pluripotent, slow self-renewing CSCs (CSC1s/TICs) would arise. CSC1s, epigenetically blocked in a ground/primed state, corresponding to the epiblast state, would form a “tumor germinal center,” continuously feeding the tumor. (4) Through autocrine/paracrine growth, from CSC1s, committed non-self-renewing secondary CSCs (CSC2s) would arise. CSC1s and CSC2s, together, would form a “tumor growth nucleus” corresponding to the epiblast/hypoblast state. (5) From CSC1s/CSC2s, tertiary CSCs (CSC3s), with a mesenchymal phenotype (EMT), would be generated. CSC3s would be able to migrate and invade adjoining sites, mimicking delamination of mesodermal precursors at the primitive streak. CSC1s, CSC2s, and CSC3s, together, would form a “tumor expansion module”. In the growing primary tumor, favorable conditions (normoxia) would induce CSC3s to proliferate asymmetrically and generate CPCs and, then, CDCs. This would determine a CSC1-CSC2-CSC3-CPC-CDC cell hierarchy and tumor progression, thus mimicking a partial, rudimentary somito-histo-organogenesis process. (6) In parallel, unfavorable conditions (hypoxia) would induce CSC3s to migrate as circulating micro-metastases, mimicking the gastrula morphogenetic movements. (7) In distant niches with proper EMT signals, CSC3s in the niche would locate as quiescent micro-metastases, mimicking embryonic locations of mesodermal cells at somitogenesis sites. (8) Micro-environmental proper MET signals, would induce mesenchymal CSC3 to revert, into epithelial self-renewing CSC1s mimicking gastrular induction (TICs). Self-renewing CSC1s, as TICs, would form a new tumor germinal center and re-initiate the tumor process in metastatic sites, repeating the same steps and reproducing the same cell types and hierarchy of the primary tumor. Tumorsphere figure was adapted from Bond et al. (2013).
