Abstract
Records of δ18O in stream flow are critical for understanding and modeling hydrological, ecological, biogeochemical and atmospheric processes. However, the number of such records are extremely limited globally and the length of such time series are usually less than a decade. This situation severely handicaps their use in model testing and evaluation. Here we present a global assessment of freshwater mollusk (bivalves & gastropods) isotope data from 25 river basins that have stream water isotope values, water temperature data and shell material isotope signatures. Our data span a latitude range of 37.50°S to 52.06°N. We show that δ18O signatures in freshwater mollusks are able to explain 95% of the variance of stream water δ18O. We use shell δ18O values and water temperature data to reconstruct stream water δ18O signatures. With freshwater mussel life expectancy ranging from a few years up to 200 years, this translation of mollusk metabolic properties into long term stream water isotope records is a promising approach for substantially extending global stream water isotope records in time and space.
Introduction
Stream water stable isotopes of oxygen and hydrogen have been used for decades in hydrological process studies on water source and age1,2. However, the vast majority of these studies have been restricted to relatively short time periods (covering a few years at best) in small experimental catchments. Long stream water isotope records, while of great value for enhancing our understanding of the water cycle in river basins, or assessing environmental and climatic changes on the continental water cycle, remain restricted to only a few large river basins as linked to the Global Network of Isotopes in Rivers (GNIR) of the International Atomic Energy Agency3–5.
Recent work has suggested that freshwater mollusks have the potential to complement the scarce stream water isotope records as living archives of in-stream environmental conditions6–10. Freshwater mollusks live in a wide variety of aquatic habitats and potentially hold information in their successive growth bands on interannual fluctuations in stream water isotope signatures over multiple decades and even centuries. For rivers in India, δ18O signatures in freshwater mollusks have been shown to follow stream water δ18O across different topographic settings10. To date, no global assessment has been undertaken to see if mollusk isotope records match streamflow isotope records across different continents, elevations, climates and hydrological regimes (e.g. from rain to snow fed rivers).
We hypothesize that shell-related δ18O signatures are a strong proxy of δ18O in stream water across a wide range of climates and hydrological regimes – offering the potential for freshwater mollusks to reconstruct historical streamwater isotope signals in global rivers. We leverage the fact that oxygen isotopes in mollusk shell material precipitates in equilibrium with water and past studies that have shown it can serve as a robust proxy for water temperature reconstruction11.
Here we present a global assessment of mollusk shell δ18O isotope signatures and their corresponding precipitation and stream water δ18O isotope data. We base this on published oxygen isotope signatures obtained from growth bands of freshwater mollusk shells (bivalves & gastropods) collected in 25 river basins (33 sampling sites; ~100 analysed aragonitic mollusk shells) with contrasting elevations and climates.
Results
From an initial list of 170 individual studies with published isotope signatures in freshwater mollusks, only 15 studies on 25 rivers and 33 sampling sites had both stream water isotope values and shell material isotope values. We relied on these 15 studies for conducting our comparative analysis (Table S1). For 22 rivers water temperature data was also available for a subsequent shell-based reconstruction of stream water δ18O signals. The mollusk sampling sites spanned a latitudinal range of 37.50°S to 52.06°N and an elevational range of 2 to 3250 m.a.s.l. Maximum elevations for the river basins ranged from 2 to 7620 m.a.s.l. and catchment areas ranged from ~75 km2 (Fleming Creek, South Carolina) to 400.000 km2 (Niger River in Africa).
In most sites, the δ18O signatures (without correction for temperature effects) in mollusks mirrored the δ18O values found in stream water, with mean values plotting close to the 1:1 line (slope of regression line = 0.82) and standard deviations below 1.5‰ (Fig. 1; Table S2). Overall, shell δ18O explained 95% of the variance of stream water δ18O. As a corollary, the δ18O signatures in mollusks largely reflected δ18O values and latitudinal gradients in precipitation – here represented by δ18O ranges in precipitation across Köppen-Geiger12 climate zones proper to each river basin (Figs S2b, S2e; Table S2). Notwithstanding regional topographic effects, δ18O signatures in freshwater mollusks appear largely controlled by the climate conditions (as defined in the Köppen-Geiger12 classification) prevailing along a latitudinal gradient in the 25 river basins – spanning from equatorial to polar climates (Fig. 1). While such relations have been suggested at specific field sites10, our study is the first to show such a relationship across catchment sizes, latitudes, elevations and ecoregions.
Figure 1.
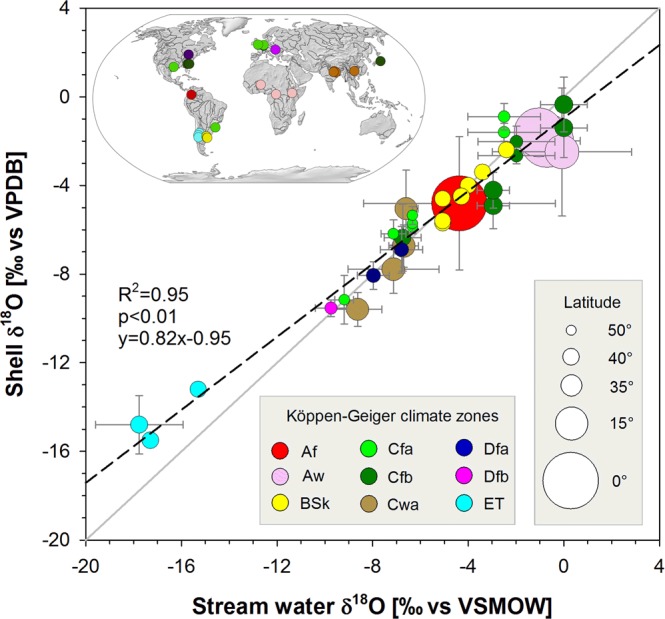
δ18O signals in stream water vs. freshwater mollusk shells [mean δ18O values with standard deviations] for 33 sampling sites across 25 river basins [map] and 9 Köppen-Geiger12 climate zones. Shell δ18O = oxygen isotopic composition of the carbonate, expressed as a deviation in ‰ from a standard carbonate, the VPDB (Vienna Pee Dee Belemnite). Water δ18O = oxygen isotopic composition of the water, expressed as a deviation in ‰ from the Vienna Standard Mean Ocean Water (VSMOW). Grey line: 1:1 line. Black dashed line: regression line (slope = 0.82). Colored dots: Köppen-Geiger12 climate zones in mollusk sampling locations ([Main climate–Precipitation–Temperature]; Af: Equatorial–fully humid; Aw: Equatorial–winter dry; BSk: Arid–summer dry–cold arid; Cfa: Temperate–fully humid–hot summer; Cfb: Temperate–fully humid–warm summer; Cwa: Temperate–winter dry–hot summer; Dfa: Cold–fully humid–hot summer; Dfb: Cold–fully humid–warm summer; ET: polar tundra. Dot size is proportional to the latitude of the shell and water sampling site. The map was created in ArcGIS version 10.5 (http://desktop.arcgis.com/) using free vector and raster map data [ne_50m_rivers_lake_centerlines; ne_110m_coastline; ne_110m_ocean; MSR_50M] made available by Natural Earth (http://naturalearthdata.com). All maps are in the public domain (http://www.naturalearthdata.com/about/terms-of-use/).
Outliers to the general pattern included sites located at higher elevations [e.g. ET Köppen climate zone], where stream water would be slightly more depleted than shell material (Fig. 1). Alternatively, sites located at lower latitudes and elevations [e.g. Aw Köppen climate zone] had stream water that tended to be slightly more enriched in comparison to shell material. In some sites, reported δ18O values in streamflow and mollusks showed large variability (as expressed by their respective standard deviations in Table S2). The Amazon River for instance, showed a very strong altitudinal gradient between the basin’s headwaters located in the Andean mountains (5220 m.a.s.l.) and the mollusk sampling site (106 m.a.s.l.). This led to a pronounced seasonality in isotopic signatures, as well as a strong altitudinal control on the depletion effect in precipitation. We hypothesize that this in turn, may lead to a strong seasonality in both stream water δ18O values (snow melt) and mollusk shell δ18O signatures – as expressed in high standard deviations of both precipitation and stream water isotope signatures (3 and 4‰, respectively; Fig. 1 & Table S2). For the Niger River, mean δ18O values in stream water and mollusk shells differed by nearly 2.5‰ and standard deviations were close to 3‰ – reflecting contrasting within-watershed climate zones, ranging from gaining rivers (defined as flow increasing with basin area) in the humid rain fed headwaters, to losing sections (defined as flow losses with increasing basin area) near the Sahara13, finally leading into more humid regions and gaining flow further downstream.
Depending on the species and the prevailing climate (temperature) and environmental (e.g. salinity) conditions, bivalves may reduce or even cease their growth during more or less prolonged periods of time14. This may eventually lead to an increasing discrepancy between shell and stream water δ18O, with shell δ18O signals exhibiting truncated patterns across potentially reduced growth periods. As a corollary this may lead to larger differences between stream water and shell δ18O signals, as noticed for both low and high latitudes in our set of 25 river basins (Fig. 1). At low latitudes, amplitudes of seasonal stream water temperature are rather small, whilst mean annual water temperatures are high (Table S2; Fig. S2a). As an example, the Oubangui River (4.21°N lat.) has a mean annual water temperature of 28.6 °C and a seasonal amplitude of stream water temperature of 6.4 °C. The rather small variability in stream water temperature (standard deviation σ = 1.2 °C for the Oubangui River) could favor the overall representativeness of δ18O values measured in shells, even in case of intermittent growth periods (caused by very high stream water temperatures, or severe low flow conditions). Towards higher latitudes, amplitudes of stream water temperature tend to increase, whilst mean annual water temperatures decrease (Fig. S2a). The Huron River (42.33°N) has a mean annual water temperature of 14.2 °C and a seasonal amplitude of stream water temperature of 27 °C. The large variability in stream water temperature (σ = 9.2 °C for the Huron River) increases the probability of a reduced representativeness of δ18O values measured from shell growth bands excluding periods with water temperatures below a species-specific threshold10,14.
Given the strong control of stream water temperature on mollusk shell growth, stream water δ18O and temperature are a common tool for reconstructing shell δ18O values14,15. We adapted this approach to reconstruct stream water δ18O for a subset of 22 rivers for which both shell mean δ18O and mean stream water temperature were available (Table S2). Our results show that estimated water δ18O closely mirrored δ18O values found in stream water (slope of the regression line = 1.00) – the reconstructed data explaining 93% of the variance of measured stream water δ18O (Fig. 2). These reconstructed stream water δ18O data reflected without any notable deviations, the difference in δ18O values of waters from different basins across a wide range of latitudes and climate settings (Table S2; Figs S2c & S2d). As expected, rivers at higher latitudes and/or elevations, with colder and wetter climates [e.g. ET Köppen climate zone], exhibited the most depleted reconstructed and measured isotope signatures; also as expected, stream water temperatures were lowest (mean annual ~5 °C) and δ18O values most depleted (~17‰ to ~15‰) in the glacier and snowmelt Andean rivers of the Mendoza province (Argentina; lat. 35–37°S). At lower latitudes the warm and drier climate conditions [e.g. Aw Köppen climate zone] signals were more enriched in reconstructed and measured isotope signatures in stream water (Figs 2; S2c,d). In African river basins, stream water temperatures were very high (e.g. Oubangui River at ~29 °C) and δ18O signatures were among the most enriched (e.g. ~0‰ in the Niger River) of our dataset (Table S2). When accounting for the variability in stream water temperature (as expressed through ± 1σ) and shell δ18O (±1σ) for calculating stream water δ18O (Fig. S2d), we found the smallest range of uncertainty for river basins located at low latitudes (e.g. ±1.2‰ for the Oubangui River). In river basins located at higher latitudes the range of uncertainty in δ18O was much larger (e.g. ±3‰ for the Huron River). A notable exception to this pattern is the Niger River, where a considerable uncertainty (±3.6‰) is triggered by the large variability in shell δ18O (σ = 2.89‰), despite a rather small seasonal stream water temperature amplitude (12.6 °C). The Amazon River equally exhibits a large uncertainty in estimated stream water δ18O (±3.3‰), with the effect of a small seasonal stream water amplitude (6.2 °C) outweighed by a large variability in shell δ18O (σ = 3.01‰).
Figure 2.
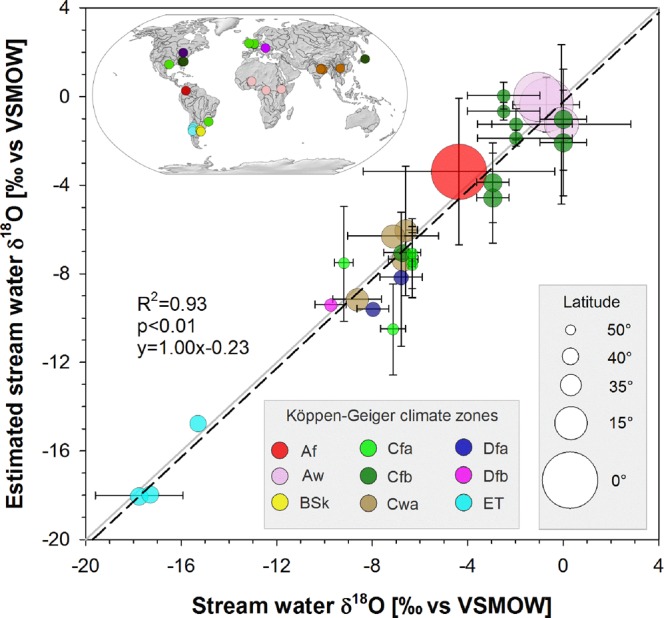
δ18O signals in stream water [mean δ18O values with standard deviations] and δ18O signals estimated (accounting for temperature effects) from shell δ18O values [mean δ18O values with standard deviations] for 22 river basins [map] and 9 Köppen-Geiger12 climate zones. Stream water δ18O = oxygen isotopic composition of the water, expressed as a deviation in ‰ from the Vienna Standard Mean Ocean Water (VSMOW). Grey line: 1:1 line. Black dashed line: regression line (slope = 1.00). Colored dots: Köppen-Geiger12 climate zones in mollusk sampling locations ([Main climate–Precipitation–Temperature]; Af: Equatorial–fully humid; Aw: Equatorial–winter dry; BSk: Arid–summer dry–cold arid; Cfa: Temperate–fully humid–hot summer; Cfb: Temperate–fully humid–warm summer; Cwa: Temperate–winter dry–hot summer; Dfa: Cold–fully humid–hot summer; Dfb: Cold–fully humid–warm summer; ET: polar tundra. Dot size is proportional to the latitude of the shell and water sampling site. The map was created in ArcGIS version 10.5 (http://desktop.arcgis.com/) using free vector and raster map data [ne_50m_rivers_lake_centerlines; ne_110m_coastline; ne_110m_ocean; MSR_50M] made available by Natural Earth (http://naturalearthdata.com). All maps are in the public domain (http://www.naturalearthdata.com/about/terms-of-use/).
Discussion
Our global assessment of mollusk shell δ18O data across a latitudinal sequence of 33 sampling sites reveals the strong links between precipitation, stream water and shell signatures – suggesting the potential for freshwater mollusks to serve as archives of past isotopic signatures in rivers over a wide range of hydro-climatological settings. Long records of δ18O series are of great value for gaining a better understanding of long-term variability and/or non-stationary hydrological, ecological, biogeochemical and atmospheric responses to global change. However, the numbers of such records remain extremely limited globally and the length of such time series are usually less than a decade. An estimated 1000 freshwater bivalve species (order Unionoida) populate a large variety of river systems and lakes around the globe. Freshwater mussels are long-lived organisms, living an average of 10 years – with many species living 20 to 30 years and some up to two centuries16 (e.g. Margaritifera margaritifera)17. Such high longevity gives them the potential for recording across their successive growth bands long-term changes in environmental conditions. While past work has used mollusks for paleo-temperature reconstructions18, our analysis shows that mollusk shells formed in isotopic equilibrium with the surrounding water11 may serve as new tools for reconstructing stream water δ18O signatures over several decades from δ18O data series measured across mollusk shell increments.
In our global assessment of mollusk shell δ18O data, four studies relied on whole-shell analyses for determining time-averaged δ18O values. Another eleven studies had analyzed successive growth increments, delivering either seasonal or inter-annual variability in δ18O signals over multiple years. A limitation to the full reconstruction of stream water δ18O data is the sensitivity of freshwater mussels to stream water temperature or salinity that may induce growth periods of different lengths depending on latitude, elevation and prevailing climate conditions. Aestivation and growth interruption may eventually cause a limited representativeness of shell δ18O data for reconstruction of annual or inter-annual stream water δ18O signatures19. Various studies on freshwater bivalves have shown that shell growth ceases at temperatures below 12 °C10,14,20. At high latitudes/elevations, shell δ18O signals exhibit an increasingly truncated sinusoidal pattern with narrow peaks (essentially due to shell growth cessation in winter) and wide troughs7. At lower latitudes/elevations, elevated water temperatures (20 to 35 °C) may substantially modify metabolic rates in freshwater mollusks and compromise biological processes such as survival, growth and reproduction21. In tropical regions with typically small temperature variations the dominant control on shell δ18O variations are the changes in stream water δ18O or monsoonal influences9,22. High discharge or low water conditions may lead to more or less prolonged growth gaps, extending up to 150 days in the Oubangui and Niger river basins9.
An additional source of uncertainty relates to the water sampling protocols applied in the 33 mollusk collection sites used in our study. While water had been sampled at, or nearby shell collection sites, sampling frequency differed strongly – ranging from unique grab samples of water to fortnightly water sampling protocols extending over several years.
The highly variable temporal resolution in shell δ18O (time-averaged vs. successive growth increments) and river water δ18O (grab samples vs. continuous sampling) across our set of 33 sampling sites resulted in substantial differences between the related standard deviations of observed and reconstructed δ18O values. Ultimately, water and mollusk shell sampling frequency, as well as individual mollusk species’ physiology, ecology and life cycle are crucial for the interpretation of observed and reconstructed isotope signatures23.
When considering the effects of mollusk shell analysis for reconstructing historical data series, it is also important to consider that aragonite is thermodynamically unstable – with a gradual recrystallization to calcite leading to a ‘resetting’ of isotopic signatures24. A thorough mineralogical assessment of the degree of preservation of aragonitic mollusk shells is therefore strongly recommended (e.g. via X-ray diffraction)25. An important next step in this context is the implementation of calibration studies – either via controlled experiments or field surveys – contributing to a better understanding of the relationships between stable isotope signals and ecological and/or environmental variables. This will eventually lead to an improved interpretation of measured variability as ecological and/or environmental ‘signal’ or stochastic ‘noise’26.
Regardless of current limitations, δ18O records from mollusk shells have the potential to open up new research avenues for quantifying climate change impacts on the long-term isotope time series of precipitation and stream water27, leading to new mechanistic understanding of processes controlling water flow and quality28, or serving for the calibration and validation of flow and transport models29,30.
Methods
We used the search terms SHELL, ISOTOP*, FRESHWATER and OXYGEN in Web of Science and found 170 individual studies on isotope signatures in mollusks. This list was reduced to 15 studies carried out on 25 rivers (lakes were ruled out), for which stable isotope data was available for both stream water and shell material (from 28 different freshwater mollusk species; Fig. S1; Table S1). Stream water temperature data was extracted from the retained list of studies, except for the regions of Northwestern Mendoza, Central-eastern Mendoza and Southern Mendoza, for which water temperature data was taken from Scheibler et al.31.
The protocols used for mollusk shell preparation, shell material sampling and isotope analyses slightly differed among the 15 studies (Table S3). Four out of the 15 studies relied on analytical protocols that consisted in crushing one or several mollusk shells and subsequent δ18O analysis of the collected shell material via a mass spectrometer (Table S3). In eleven studies shell annual growth bands were sampled either with micromilling devices, dental drills, or scalpel blades. The collected shell material was then analyzed with a mass spectrometer. Secondary Ion Mass Spectrometry has been recently used for obtaining high-resolution records of isotope signatures in mollusk shells32–34. Prior to isotope analysis, growth bands are identified through dyeing with Mutvei’s solution.
For the 33 mollusk sampling sites of our global assessment, δ18O values were either integrated (i.e. obtained via whole-shell analyses) or spanning over successive growth increments (i.e. years of growth bands). For an individual site, mean values either correspond to an average of δ18O values from several shells or to an average of δ18O values sampled across shell growth increments.
For converting δ18O in shell material to δ18O in stream water, the reported studies relied on equations relating water temperature and δ18O in stream water (e.g. Dettman et al.14, Gonfiantini et al.15, Friedman and O’Neil35), such as:
| 1 |
where T = stream water temp. (in °K) and α = fractionation between water and aragonite
| 2 |
Since δ18Oar values are first made relative to the Vienna Pee Dee Belemnite (VPDB) reference, they are commonly converted to the Vienna Standard Mean Ocean Water (VSMOW) as per Gonfiantini et al.15:
| 3 |
On the basis of equations (1) and (2) we were able to estimate stream water δ18O from shell δ18O and stream water temperature:
| 4 |
We extracted the isotopic values in stream water and mollusk shells and stream water temperature data from the primary literature via the data extraction software PlotDigitizer, as well as from tables (Tables S1–S3).
For the 25 river basins, we relied on the Köppen-Geiger climate zones as per Rubel and Kottek12 to determine δ18O ranges in precipitation (Table S2 and Fig. S2).
Supplementary information
Acknowledgements
We thank Núria Martínez-Carreras for preparing the world maps with locations of isotopes in freshwater mussel studies. Stan Schymanski and Ryan Teuling provided valuable comments on an earlier version of the manuscript. We thank Kim Janzen for editing assistance. Part of this work was funded through the National Research Fund of Luxembourg (grant INTER Mobility/15/10948104 JEDI).
Author Contributions
L.P. has designed the study, contributed to the data analysis and interpretation and wrote the manuscript. C.G. has extracted the stable isotope datasets from the 15 retained studies and contributed to data analysis. J.N.B. and J.M.D. participated in the data analysis, interpretation of data and results, as well as in manuscript revisions.
Data Availability
The datasets are available from the individual studies retained for this work, as well as from the authors of this manuscript upon request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40369-0.
References
- 1.Klaus J, McDonnell JJ. Hydrograph separation using stable isotopes: Review and evaluation. Journal of Hydrology. 2013;505:47–64. doi: 10.1016/j.jhydrol.2013.09.006. [DOI] [Google Scholar]
- 2.Sprenger M, Volkman THM, Blume T, Weiler M. Estimating flow and transport parameters in the unsaturated zone with pore water stable isotopes. Hydrology and Earth System Sciences. 2015;19:2617–2635. doi: 10.5194/hess-19-2617-2015. [DOI] [Google Scholar]
- 3.IAEA. Monitoring isotopes in rivers: creation of the global network of isotopes in rivers (GNIR). IAEA-TECDOC-1673. ISBN 978-92-0-126810-5. 258 pages (IAEA, 2012).
- 4.Vitvar T, Aggarwal PK, Herczeg AL. Global network is launched to monitor isotopes in rivers. Eos, Transactions, American Geophysical Union. 2007;88:325–332. doi: 10.1029/2007EO330001. [DOI] [Google Scholar]
- 5.Halder J, Terzer S, Wassenaar LI, Araguás-Araguás LJ, Aggarwal PK. The Global Network of Isotopes in Rivers (GNIR): integration of water isotopes in watershed observation and riverine research. Hydrology and Earth System Sciences. 2015;19:3419–3431. doi: 10.5194/hess-19-3419-2015. [DOI] [Google Scholar]
- 6.Helama S, Nielsen JK. Construction of statistically reliable sclerochronology using subfossil shells of river pearl mussel. Journal of Paleolimnology. 2008;40:247–261. doi: 10.1007/s10933-007-9155-5. [DOI] [Google Scholar]
- 7.Versteegh E, Troelstra S, Vonhof H, Kroon D. Oxygen isotope composition of bivalve seasonal growth increments and ambient water in the rivers Rhine and Meuse. PALAIOS. 2009;24:497–504. doi: 10.2110/palo.2008.p08-071r. [DOI] [Google Scholar]
- 8.Versteegh, E., Vonhof, H. B., Troelstra, S. R., Kaandorp, R. J. G. & Kroon, D. Seasonally resolved growth of freshwater bivalves determined by oxygen and carbon isotope shell chemistry. Geochemistry, Geophysics, Geosystems. 11 (2010).
- 9.Kelemen Z, et al. Calibration of hydroclimate proxies in freshwater bivalve shells from Central and West Africa. Geochimica et Cosmochimica Acta. 2017;208:41–62. doi: 10.1016/j.gca.2017.03.025. [DOI] [Google Scholar]
- 10.Gajurel AP, France-Lanord C, Huyghe P, Guilmette C, Gurung D. C and O isotope compositions of modern fresh-water mollusc shells and river waters from the Himalaya and Ganga plain. Chemical Geology. 2006;233:156–183. doi: 10.1016/j.chemgeo.2006.03.002. [DOI] [Google Scholar]
- 11.Urey, H. C. The thermodynamic properties of isotopic substances. Journal of the Chemical Society, 562–581 (1947). [DOI] [PubMed]
- 12.Rubel F, Kottek M. Observed and projected climate shifts 1901–2100 depicted by world maps of the Köppen-Geiger climate classification. Meteorologische Zeitschrift. 2010;19:135–141. doi: 10.1127/0941-2948/2010/0430. [DOI] [Google Scholar]
- 13.Ingraham, N. L., Caldwell, E. A. & Verhagen, B. Th. Arid catchments. In Isotope tracers in catchment hydrology (Elsevier, 1998).
- 14.Dettman DL, Reische AK, Lohmann KC. Controls on the stable isotope composition of seasonal growth bands in aragonitic fresh-water bivalves (unionidae) Geochimica et Cosmochimica Acta. 1999;63:1049–1057. doi: 10.1016/S0016-7037(99)00020-4. [DOI] [Google Scholar]
- 15.Gonfiantini, R., Kozdon, J. & Peters, S. E. Standards and intercomparison materials distributed by the International Atomic Energy Agency for stable isotope measurements. Reference and intercomparison materials for stable isotopes of light elements. IAEA-TECDOC-825. 13–29 (IAEA, 1995).
- 16.Anthony JL, Kesler DH, Downing WL, Downing JA. Length-specific growth rates in freshwater mussels (Bivalvia: Unionidae): extreme longevity or generalized growth cessation? Freshwater Biology. 2001;46:1349–1359. doi: 10.1046/j.1365-2427.2001.00755.x. [DOI] [Google Scholar]
- 17.Dunca E, Söderberg H, Norrgrann O. Shell growth and age determination in the freshwater pearl mussel Margaritifera margaritifera in Sweden: natural versus limed streams. Ferrantia. 2011;64:48–58. [Google Scholar]
- 18.Goodwin DH, Schöne BR, Dettman DL. Resolution and fidelity of oxygen isotopes as paleotemperature proxies in bivalve mollusk shells: models and observations. PALAIOS. 2003;18:110–125. doi: 10.1669/0883-1351(2003)18<110:RAFOOI>2.0.CO;2. [DOI] [Google Scholar]
- 19.Leng MJ, Lewis JP. Oxygen isotopes in Molluscan shell: applications in environmental archaeology. Environmental Archaeology. 2016;21:295–306. doi: 10.1179/1749631414Y.0000000048. [DOI] [Google Scholar]
- 20.Caroll M, Romanek C, Paddock L. The relationship between the hydrogen and oxygen isotopes of freshwater bivalve shells and their home streams. Chemical Geology. 2006;234:211–222. doi: 10.1016/j.chemgeo.2006.04.012. [DOI] [Google Scholar]
- 21.Ganser AM, Newton TJ, Haro RJ. Effects of elevated water temperature on physiological responses in adult freshwater mussels. Freshwater Biology. 2015;60:1705–1716. doi: 10.1111/fwb.12603. [DOI] [Google Scholar]
- 22.Kaandorp RJG. Seasonal stable isotope variations of the modern Amazonian freshwater bivalve Anodontites trapesialis. Palaeogeography, Palaeoclimatology, Palaeoecology. 2003;194:339–354. doi: 10.1016/S0031-0182(03)00332-8. [DOI] [Google Scholar]
- 23.Shanahan TM, Pigati JS, Dettman DL, Quade J. Isotopic variability in the aragonite shells of freshwater gastropods living in springs with nearly constant temperature and isotopic composition. Geochimica et Cosmochimica Acta. 2005;69:3949–3966. doi: 10.1016/j.gca.2005.03.049. [DOI] [Google Scholar]
- 24.Leng MJ, Marshall JD. Palaeoclimate interpretation of stable isotope data from lake sediment archives. Quaternary Science Reviews. 2004;23:811–831. doi: 10.1016/j.quascirev.2003.06.012. [DOI] [Google Scholar]
- 25.Leng MJ, Lamb AL, Lamb HF, Telford RJ. Palaeoclimatic implications of isotopic data from modern and early Holocene shells of the freshwater snail Melanoides tuberculata, from lakes in the Ethiopian Rift Valley. Journal of Paleolimnology. 1999;21:97–106. doi: 10.1023/A:1008079219280. [DOI] [Google Scholar]
- 26.Van Hardenbroek M, et al. The stable isotope composition of organic and inorganic fossils in lake sediment records: Current understanding, challenges, and future directions. Quarternary Science Reviews. 2018;196:154–176. doi: 10.1016/j.quascirev.2018.08.003. [DOI] [Google Scholar]
- 27.Rank D, Wyhlidal S, Schott K, Weigand S, Oblin A. Temporal and spatial distribution of isotopes in river water in Central Europe: 50 years experience with the Austrian network of isotopes in rivers. Isotopes in Environmental and Health Studies. 2018;54:115–136. doi: 10.1080/10256016.2017.1383906. [DOI] [PubMed] [Google Scholar]
- 28.Darling WG, Bowes MJ. A long-term study of stable isotopes as tracers of processes governing water flow and quality in a lowland river basin: the upper Thames, UK. Hydrological Processes. 2016;30:2178–2195. doi: 10.1002/hyp.10779. [DOI] [Google Scholar]
- 29.Reckert A, Stichler W, Schmidt A, Stumpp C. Long-term data set analysis of stable isotopic composition in German rivers. Journal of Hydrology. 2017;552:718–731. doi: 10.1016/j.jhydrol.2017.07.022. [DOI] [Google Scholar]
- 30.Risi C, et al. The water isotopic version of the land-surface model ORCHIDEE: Implementation, evaluation, sensitivity to hydrological parameters. Hydrology Current Research. 2016;7:1000258. doi: 10.4172/2157-7587.1000258. [DOI] [Google Scholar]
- 31.Scheibler EE, Claps MC, Roig-Juñent SA. Temporal and altitudinal variations in benthic macroinvertebrate assemblages in an Andean river basin of Argentina. Journal of Limnology. 2014;73:92–108. doi: 10.4081/jlimnol.2014.789. [DOI] [Google Scholar]
- 32.Linzmeier BJ, Kozdon R, Peters SE, Valley JW. Oxygen isotope variability within Nautilus shell growth bands. PLoS One. 2016;11:e0153890. doi: 10.1371/journal.pone.0153890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vihtakari M, et al. Decoding the oxygen isotope signal for seasonal growth patterns in Arctic bivalves. Palaeogeography, Palaeoclimatology, Palaeoecology. 2016;446:263–283. doi: 10.1016/j.palaeo.2016.01.008. [DOI] [Google Scholar]
- 34.Pfister, L. et al. Freshwater pearl mussels as a stream water stable isotope recorder. Eco-hydrology, e2007 (2018).
- 35.Friedman, I. & O’Neil, J. R. Data of geochemistry – Compilation of stable isotope fractionation factors of geochemical interest. Geological Survey Professional Paper 440-KK (1977).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets are available from the individual studies retained for this work, as well as from the authors of this manuscript upon request.


