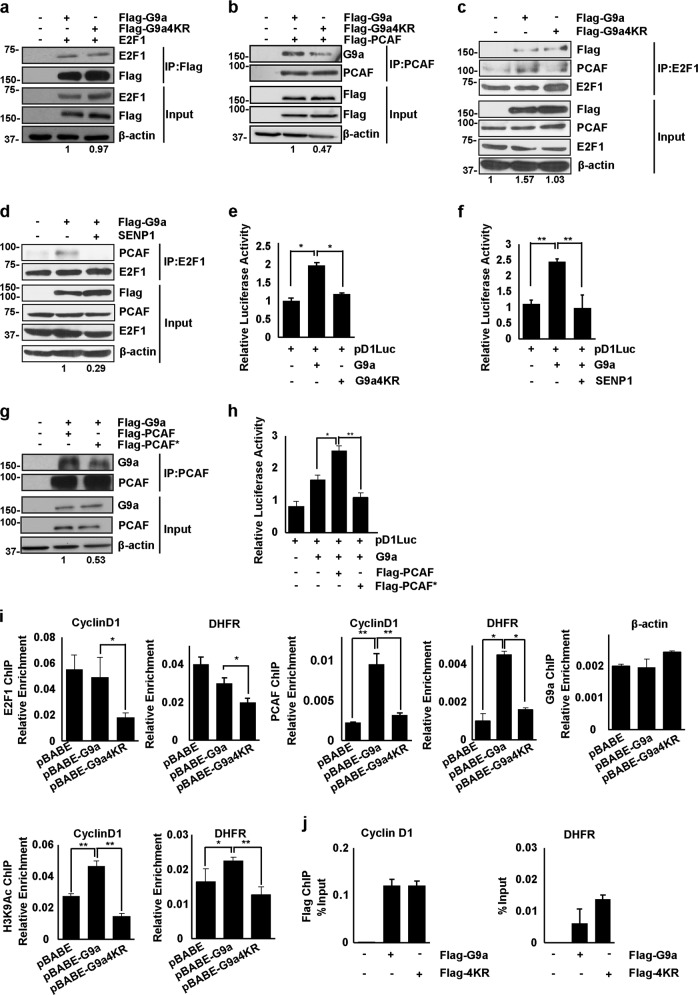
Fig. 4. G9a SUMOylation regulates E2F1/PCAF (P300/CBP-associated factor) association.
a HEK293T cells were co-transfected with E2F1 and Flag-G9a or Flag-G9a4KR. Lysates were immunoprecipitated with Flag-agarose beads and immunoblotted with anti-E2F1, anti-G9a, and anti-β-actin antibodies. Numbers at the bottom of the panel indicate the relative interaction of E2F1 with G9a and G9a4KR, which was quantified by calculating the ratio of E2F1 to Flag band intensities in the immunoprecipitated material. The interaction with G9a was given a value of 1. b HEK293T cells were co-transfected with PCAF and Flag-G9a or Flag-G9a4KR. Lysates were immunoprecipitated with anti-PCAF antibody and immunoblotted with anti-PCAF, anti-G9a, and anti-β-actin antibodies. The relative interaction of PCAF with G9a and G9a4KR was quantified by calculating the ratio of G9a to PCAF band intensities in the immunoprecipitated material. The interaction with G9a was given a value of 1. c C2C12 cells were transfected with Flag-G9a or Flag-G9a4KR. Lysates were immunoprecipitated with anti-E2F1 antibody and immunoblotted with anti-PCAF and anti-FLAG antibodies. The ratio of PCAF to E2F1 band intensities was quantified in the immunoprecipitated material. Numbers at the bottom of the panel show interaction relative to control cells which was given a value of 1. d Flag-G9a was transfected with or without SENP1 as indicated. Lysates were immunoprecipitated with anti-E2F1 antibody and immunoblotted with anti-PCAF, anti-E2F1, and anti-β-actin antibodies. The band intensities of PCAF with E2F1 were quantified in the immunoprecipitated material. The interaction in the presence of G9a was given a value of 1. e Cells stably expressing pBABE-G9a or pBABE-G9a4KR were transfected with 200 ng of Cyclin D1 promoter (pD1luc) reporter. After 48 h, the luciferase activity was measured using dual luciferase reporter assays. f pBABE and pBABE-G9a cells were transfected with 200 ng of Cyclin D1 promoter (pD1luc) with or without SENP1. Luciferase activity was measured using dual luciferase reporter assays. g HEK293T cells were co-transfected with Flag-G9a and PCAF or PCAF-SUMO interaction motif (SIM) mutant (PCAF*). Lysates were immunoprecipitated with anti-PCAF antibody, followed by immunoblotting with anti-PCAF, anti-G9a, and anti- β-actin antibodies. The interaction of PCAF and PCAF* with G9a was quantified by calculating the ratio of G9a to PCAF band intensities in the immunoprecipitated material. Numbers at the bottom of the panel indicate interaction relative to PCAF that was given a value of 1. h pBABE and pBABE-G9a cells were co-transfected with 200 ng of Cyclin D1 reporter along with PCAF or PCAF SIM mutant (PCAF*). Luciferase activity was measured using dual luciferase reporter assays. i Chromatin immunoprecipitation (ChIP) assays were performed with anti-E2F1, anti-PCAF, and anti-H3K9ac antibodies at Cyclin D1 and dihydrofolate reductase (DHFR) promoters in proliferating pBABE, pBABE-G9a, or pBABE-G9a4KR cells. As a negative control, G9a ChIP was performed at the β-actin promoter in pBABE, pBABE-G9a, and pBABE-G9a4KR cells. j No change in G9a occupancy was seen in Flag-G9a and Flag-G9a4KR cells using anti-Flag antibody. .Significance was determined using Student’s t-test (*p < 0.05, **p < 0.001, ****p < 0.001).