Figure 1.
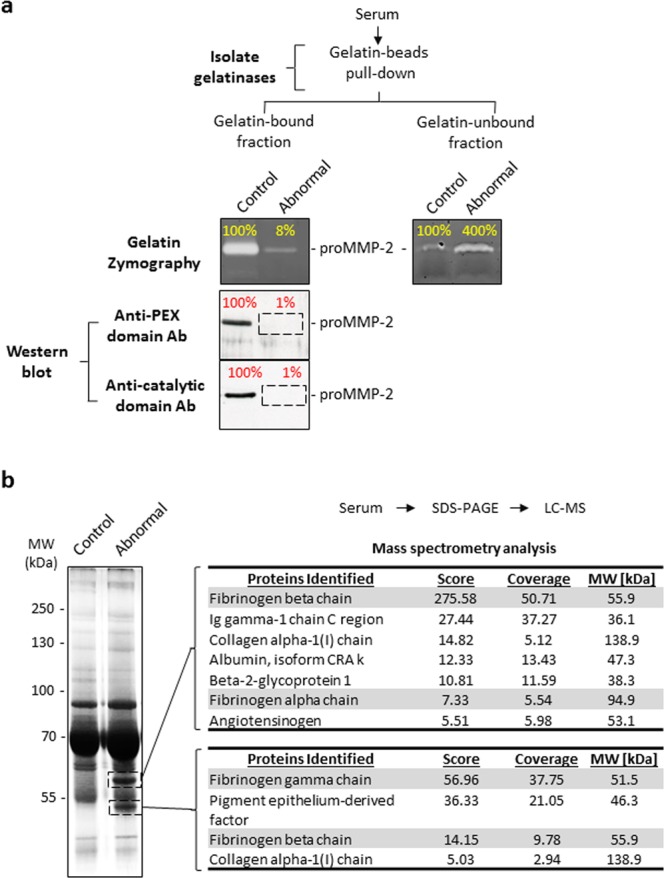
Identification of a blood donor with elevated serum fibrinogen exhibiting impaired binding of MMP-2 to gelatin. (a) Analysis of serum samples by gelatin zymography and Western blot to detect MMP-2 and/or to quantitate its gelatinolytic activity. Undiluted serum samples (10 μL) were incubated with an estimated 43 μg of immobilized gelatin at 4 °C for 1 hour. Gelatin-bound and gelatin-unbound proteins were subjected to gelatin zymography to quantitate MMP-2 activity. Gelatin-bound proteins were subjected to Western blot to detect MMP-2 using anti-PEX and anti-catalytic domain (MMP-2) antibodies. Top: Gelatin zymogram showing the majority of activity of serum MMP-2 of the abnormal sample remained in the gelatin-unbound fraction. Bottom: Western blots showing serum MMP-2 of the abnormal sample was undetected in the gelatin-bound fraction by both the anti-PEX domain and anti-catalytic domain antibodies. Band intensities are shown as a percentage relative to the band of the representative control serum sample. Uncropped gels and blots are presented in Supplementary Fig. S7. (b) SDS-PAGE coupled with LC-MS (ESI) was used to identify the compositions of two unique protein bands in the abnormal sample as indicated. Uncropped gel is presented in Supplementary Fig. S7. LC-MS, liquid chromatography – mass spectrometry; ESI, electrospray ionization; PEX, hemopexin-like; Ab, antibody; MMP-2, matrix metalloproteinase 2.
