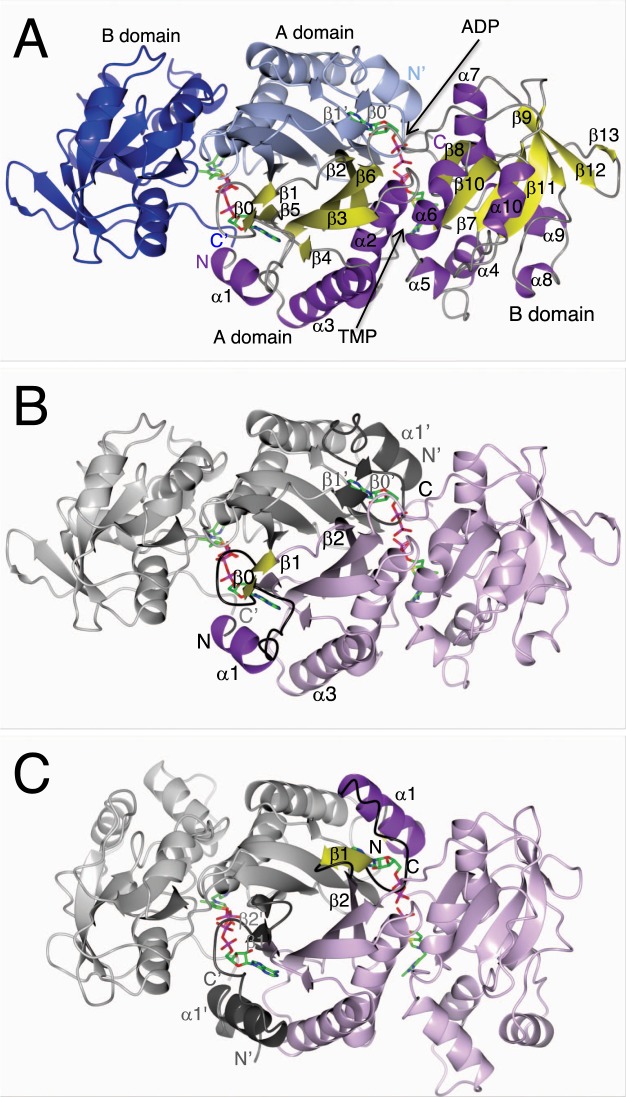
Figure 1.
Dimer of ThiL. (A) shows the dimer of AbThiL bound to ADP/TMP looking down a dyad. The model of chain A on the right side is colored by secondary structure elements, helices in purple, strands in yellow. The model of chain B on the left side is colored by domains, the A domain in light blue, the B domain in blue. Strands β0′ and β1′ are part of the β-sheet that is mostly located in domain A. (B,C) highlight the domain swap of the N-terminus. In (B), the dimer of AbThiL is shown in the same orientation as in. (A) Chain A is colored in light purple with secondary structure elements α1, β0 and β1 highlighted in purple and yellow, respectively, and loops between them in black. The corresponding region in chain B is marked in dark grey. In (C), the model of AaThiL is shown in the same orientation and the same color scheme. Helix α1 and strand β1 of AaThiL are located in the space that helix α1′ and strand β1′ occupy in AbThiL. Strand β0 is unique to AbThiL and part of a loop in AaThiL. Starting with strand β2 secondary structure elements match up again.