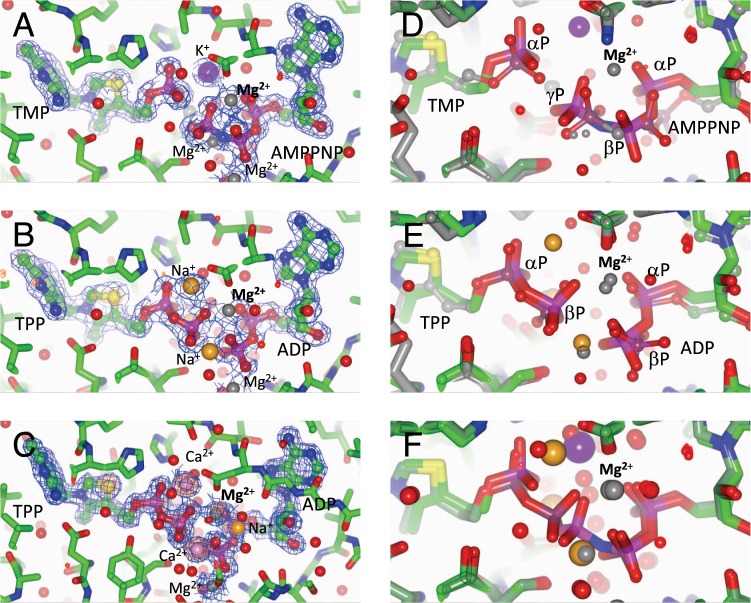
Figure 2.
Active site of ThiL. Panels A–C show an overview of the active site and the bound ligands for AbThiL. Panels D–F zoom into the reaction site and compare AbThiL with AaThiL (D/E), and the product and substrate complex for AbThiL. (F) Metals are colored as follows: Mg2+ grey, Ca2+ pink, Na+ orange, K+ purple. (A) AbThiL with TMP and AMPPNP, oP form, 2Fo-Fc map at 1 σ (5DD7). (B) AbThiL with TPP and ADP, oP form, 2Fo-Fc map at 1 σ, anomalous map at 3 σ (5CM7). (C) AbThiL with TPP and ADP, mP form, 2Fo-Fc map at 1 σ, anomalous map25 at 3 σ (5D9U). (D) AbThiL with TMP and AMPPNP (ANP) in thick and bright, and AaThiL with TMP and AMPPCP (thin, dark, 3C9T), note different orientation of the α-phosphate groups of TMP. (E) AbThiL with ADP and TDP (thick, bright) and AaThiL with ADP and TPP (thin, dark, 3C9U), note the similar orientation of phosphate groups. (F) AbThiL with AMPPNP (ANP) and TMP (dark) and ADP and TPP (bright), suggesting an in-line transfer of the phosphate group. The invariable Mg2+ ion that either bridges the scissile phosphorester bond between β-phosphate and γ-phosphate in the substrate complex, or that bridges between β-phosphate of TPP and β-phosphate in ADP in the product complex is highlighted in bold.