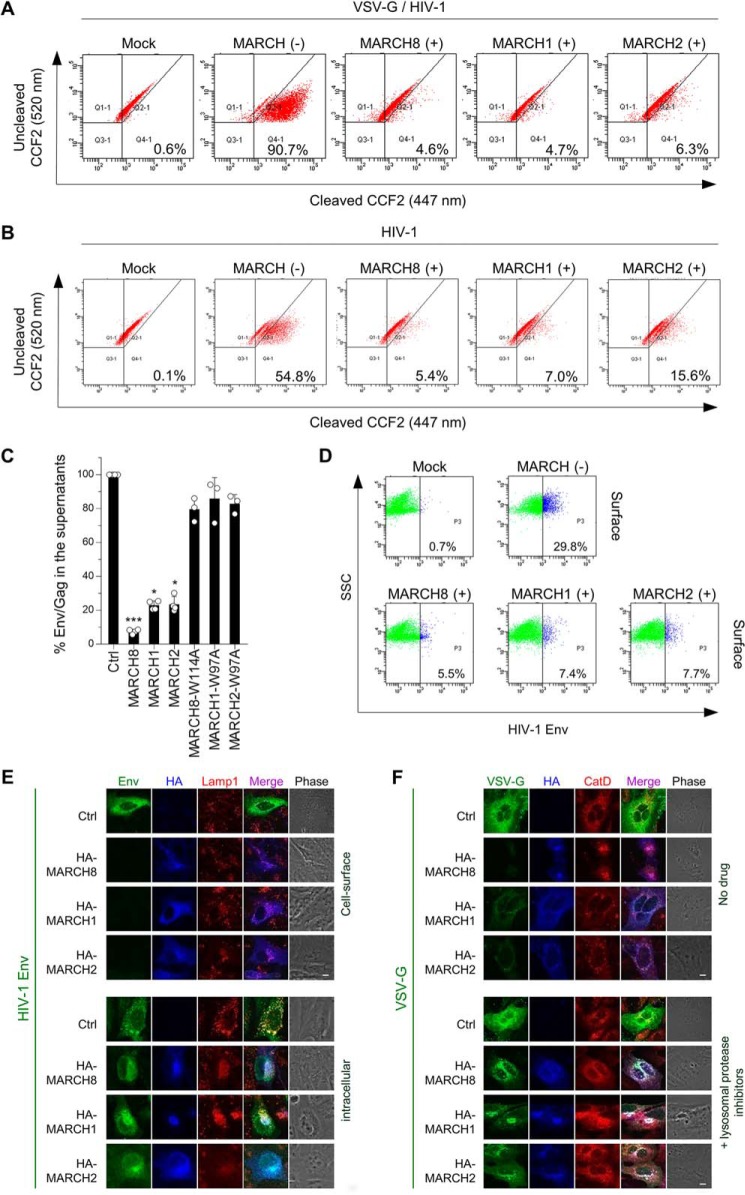
Figure 3.
A and B, MARCH1, MARCH2, and MARCH8 reduce viral entry efficiency by blocking virion incorporation of envelope glycoproteins. Shown are BlaM-Vpr–based viral entry assays using VSV-G–pseudotyped viruses (A) or NL4–3 whole viruses (B) produced from cells expressing MARCH proteins or the vector control. Representative FACS dot plots from four independent experiments are shown. C, expression of MARCH1, MARCH2, and MARCH8 in producer cells decreases HIV-1 gp120 levels in viral supernatants. Shown are ELISA-based levels of Env gp120 in viral supernatants from 293T cells cotransfected with luc reporter proviral DNA and the NL-Env plasmid together with the MARCH expression plasmid. Data are shown as the percent of gp120 Env/p24Gag in the supernatants relative to that from control (Ctrl) cells (mean ± S.D. from at least three independent experiments). *, p < 0.05; ***, p < 0.005 compared with the control vector using one-way analysis of variance and Dunn's multiple comparisons test. D–F, MARCH1, MARCH2, and MARCH8 down-regulate envelope glycoproteins on the cell surface. Shown are FACS-based (D) and immunofluorescence-based (E and F) analyses of cell surface (D and E, top) and intracellular levels of HIV-Env (E, bottom) and VSV-G (F) with or without MARCH proteins in transfected HOS cells. Notably, for cell surface staining of HIV-1 Env (E, top), MARCH proteins were stained after permeabilization. VSV-G was rescued from MARCH8-induced degradation in the presence of lysosomal protease inhibitors. CatD, cathepsin D. Scale bars = 10 μm.