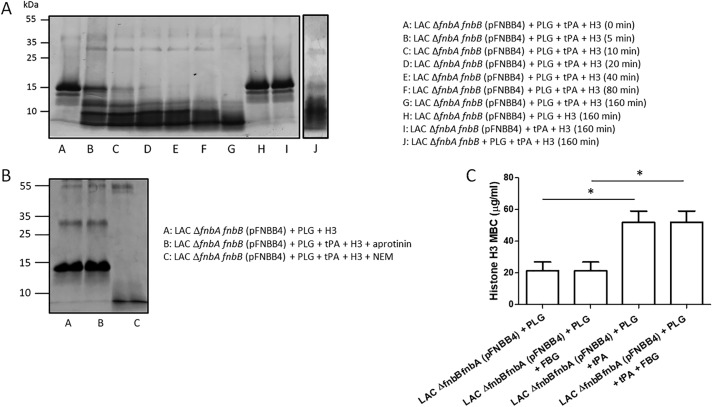
Figure 12.
Specificity of H3 cleavage by bacteria-bound plasmin. A, cells of S. aureus LAC ΔfnbAfnbB mutant overexpressing FnBPB were immobilized on the surface of microtiter plates and then incubated with human PLG. After washing, t-PA and histone H3 were added to the wells and incubated for increasing periods of time. Supernatants were subjected to SDS-PAGE and the gels stained with Coomassie Blue. Controls made of mixtures without PLG or t-PA are reported. The effect of activated PLG captured by the ΔfnbAfnbB mutant cells on H3 cleavage is also reported. The figure is representative of two independent experiments. B, to demonstrate the specificity of proteolysis, immobilized bacteria were incubated with PLG, and t-PA and H3 were added and further incubated in the presence of aprotinin or NEM. Supernatants were then analyzed for H3 cleavage by SDS-PAGE. C, cells of the S. aureus LAC ΔfnbAfnbB mutant overexpressing FnBPB were mixed with human PLG, added to serial 1.3-fold diluted histone H3 in the presence/absence of t-PA, and incubated for additional periods of time. The MBC of histone H3 was determined by plating serial dilutions of bacteria on BHI agar. To analyze the effect of FBG on H3 cleavage by activated PLG, bacteria preincubated with PLG were mixed with equimolar concentrations of H3 and FBG and processed as above. The reported data are the mean values ± S.D. from three independent experiments. Statistically significant differences are indicated (Student's t test; *, p < 0.05).