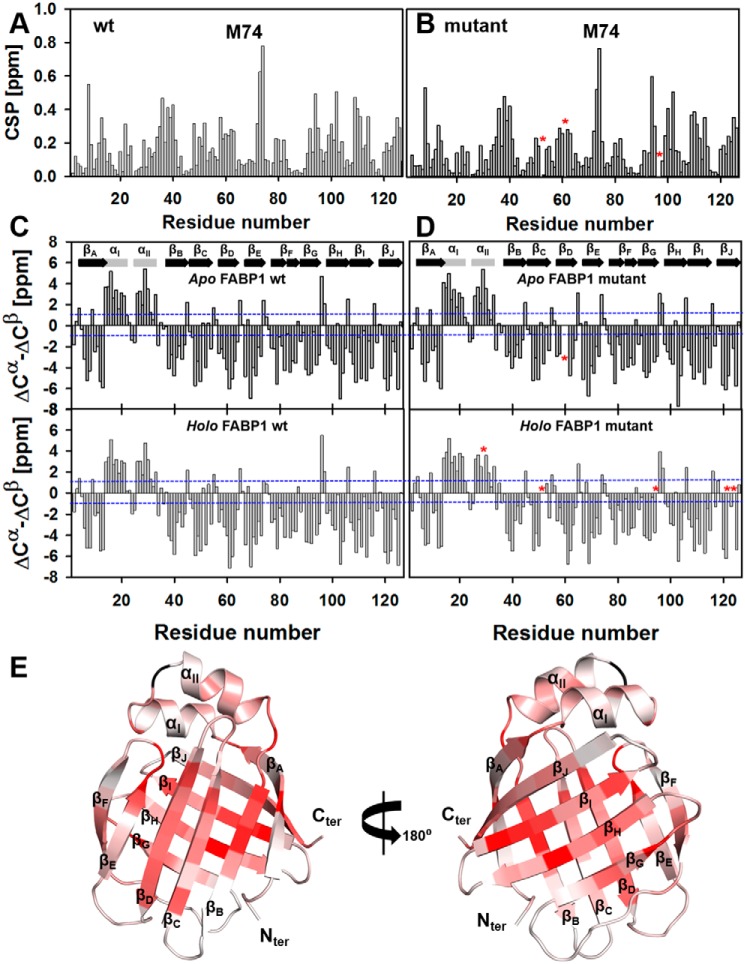
Figure 3.
Chemical shift perturbations observed upon addition of GW7647 to FABP1 and secondary shift analysis. CSP observed for WT FABP1 (A) and K57A/E77A/K96A triple-mutant FABP1 (B) plotted against amino acid residue number. Missing or ambiguous assignments are highlighted in each panel with an asterisk. In each case, the largest CSP on GW7647 binding was observed for Met-74, which is labeled. Secondary chemical shifts (ΔCα − ΔCβ) were plotted against amino acid residue number for WT (C) and triple-mutant (D) FABP1. ΔCα and ΔCβ represent the difference between observed and random coil Cα and Cβ chemical shifts, respectively. Blue dotted lines indicate the secondary shifts either of +1 or −1 ppm. Regular secondary structure elements observed for WT FABP1 are indicated on the top panels. E, cartoon representation of the structure of FABP1 colored by the extent of CSP. The cartoon is colored from light to dark red on a ramp with dark red representing a perturbation of ≥0.5 ppm. Regular secondary structure elements are labeled.