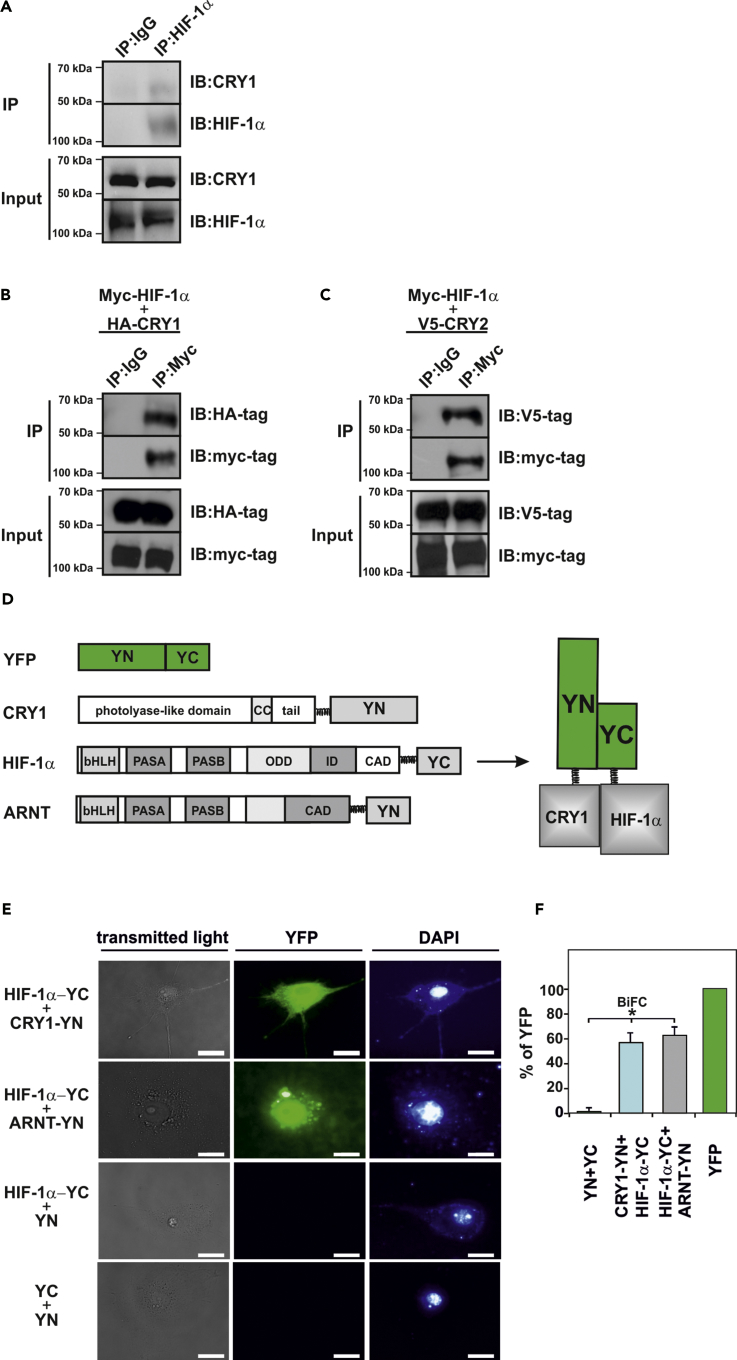
Figure 4.
CRY1 Interacts with HIF-1α
(A) Coimmunoprecipitation (IP) assays from wild-type MEFs cultured under hypoxia in the presence of MG-132. Blots from anti-HIF-1α IPs were probed with the CRY1 and HIF-1α antibody. The blots shown are representative of three independent experiments. IB, immunoblotting.
(B and C) Coimmunoprecipitation studies with HEK293 cells, expressing myc-tagged HIF-1α with either (B) HA-tagged CRY1 or (C) V5-tagged CRY2. Blots from anti-myc-tag IPs were probed with HA-tag or V5-tag antibody. The blots shown are representative of three independent experiments.
(D and E) BiFC analysis. (D) Schematic representation of expressed proteins employed in BiFC. The sequences encoding amino acid residues 1–154 (N-terminus [YN]) or 155–238 (C-terminus [YC]) of the yellow fluorescent protein (YFP) were fused to the 3′ ends of the coding regions for HIF-1α, CRY1, or ARNT to generate HIF-1α -YC, CRY1-YN, and ARNT-YN, respectively. YN and YC are non-fluorescent fragments that do not interact per se with each other. An interaction between the proteins fused to the YN and YC fragments facilitates their association to form a bimolecular fluorescent complex. (E) Visualization of the BiFC signal by confocal microscopy. COS-7 cells cotransfected with the expression vectors for HIF-1α-YC, CRY1-YN, ARNT-YN, and vectors encoding only YN or YC were cultured on glass slides for 24 h. The fluorescence detection was performed using specific filter sets for YFP and DAPI. Scale bar, 10 μm.
(F) Quantification of the BiFC signal. Cells were transfected as in (E) and quantified by flow cytometry (cf. Transparent Methods). The cytomegalovirus promoter (CMV) driven-YFP signal was set to 100%. Data are mean ± SEM. *p < 0.05.