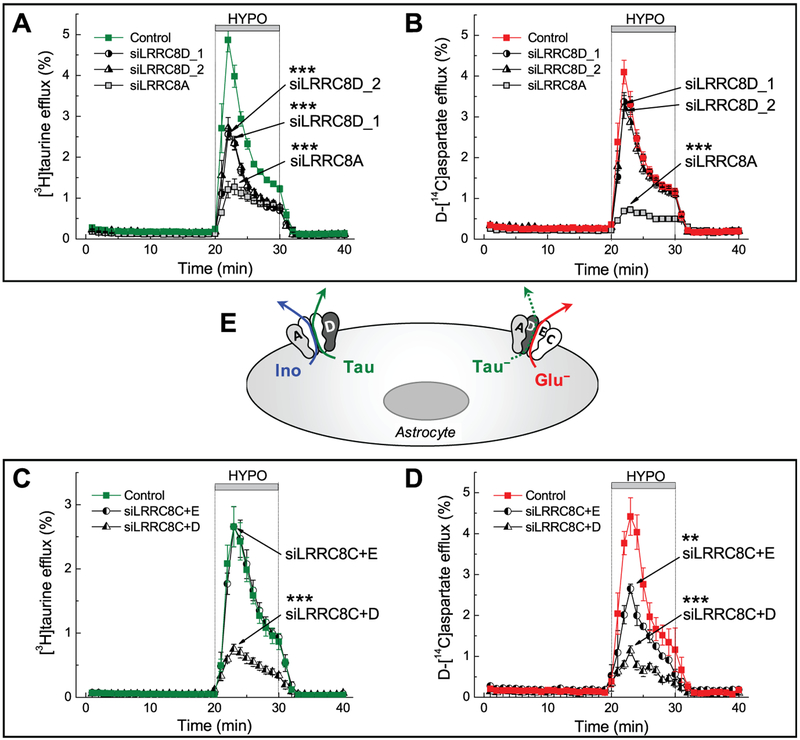
Figure 12.7.
RNAi analysis reveals that astrocytes express two types of heteromeric LRRC8-containing VRAC channels with differential permeability to charged and uncharged organic osmolytes. (A–D) Primary rat astrocytes were transfected with negative control siRNA (Control), or the gene-specific siRNA targeting the expression of one or more members of the proteins belonging to the family of leucine-rich repeat-containing 8 (LRRC8) proteins. After a 4-day incubation with the gene specific siRNA constructs, astrocytes were simultaneously loaded with [3H]taurine and the glutamate analog D-[14C]aspartate, and superfused with isosmotic or hypoosmotic (HYPO) media to activate VRAC and quantify the amino acid release. Taurine and glutamate fluxes have been measured simultaneously but, for clarity, are presented in separate panels (A-B) and (C-D). Knockdown of LRRC8A reduced release of all amino acids. Knockdown of LRRC8D reduced [3H]taurine release by ~50% (A), but had no effect on the release of D-[14C]aspartate (B). Conversely, the combined knockdown of LRRC8C + LRRC8E inhibited the efflux of D-[14C]aspartate (D), but not [3H]taurine (C). Finally, and surprisingly, the combined knockdown of LRRC8C + LRRC8D inhibited the release of all amino acids to the same extent as the knockdown of LRRC8A. (E) The proposed composition of the two populations of LRRC8-containing VRAC heteromers which explains the data in (A-D). The LRRC8A+D combination is responsible for the movement of uncharged molecules such as taurine and myo-inositol (Ino) (data not shown). Another channel composed of LRRC8A+C+D+E is permeable to negatively charged molecules, including glutamate. Because zwitterionic taurine is partially negatively charged, it shares both pathways. **p<0.01, ***p<0.001, amino acid release rates vs. cells treated with the negative control siRNA. Adapted from A.L. Schober et al. (2017), with permission.