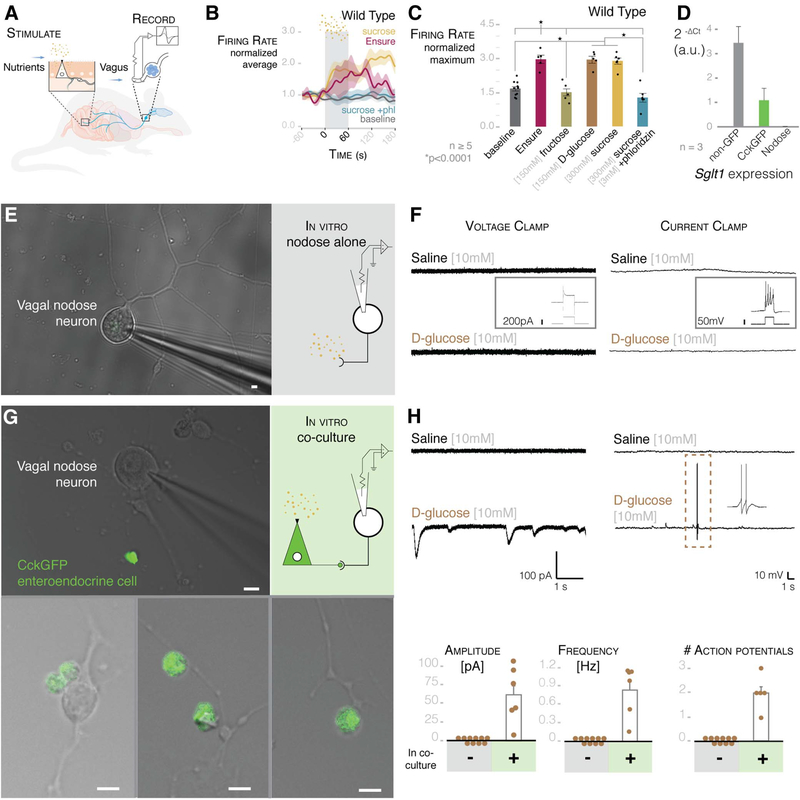
Fig. 3. Enteroendocrine cells transduce glucose stimuli onto vagal neurons.
(A) Model of intestinal intraluminal perfusion and vagal nerve electrophysiology. (B) Normalized traces for baseline, Ensure, 300 mM sucrose, and 300 mM sucrose with 3 mM phloridzin (phl) in wild-type mice. Gray bar indicates treatment period; shading indicates SEM. (C) Ensure, 300 mM sucrose, and 150 mM D-glucose stimulate vagal firing rate, which is abolished by SGLT1-blocker phloridzin [n ≥ 5 mice; *P < 0.0001, analysis of variance (ANOVA) with post hocTukey’s HSD test; error bars indicate SEM]. (D) Intestinal epithelial cells express Sgltl, but nodose neurons do not (n = 3 mice, >10,000 cells per cell type per mouse; data are presented as mean ± SEM). (E) Nodose neurons cultured alone for electrophysiology (widefield microscopy image on left, model on right). (F) Nodose neurons do not respond to 10 mM glucose in voltage-clamp (left trace) or current-clamp (right trace) mode. Insets show that neurons respond to voltage or current pulse, indicating viability. (G) Nodose neurons cocultured with GFP-positive enteroendocrine cells for electrophysiology (image on left, model on right). Innervated enteroendocrine cells are shown at the bottom. (H) In coculture, glucose evoked EPSCs (top left) and action potentials (top right) in connected neurons (scale of current or voltage and time are shown below the traces). Dashed-line box indicates action potentials expanded in right inset. Quantification of EPSC amplitude and frequency (bottom left and center; n = 21 neurons alone; n = 6 neurons connected to enteroendocrine cells) and action potentials (bottom right; n = 21 alone; n = 5 neurons connected to enteroendocrine cells) in GFP-negative (−) and -positive (+) cells. All scale bars, 10 μm.