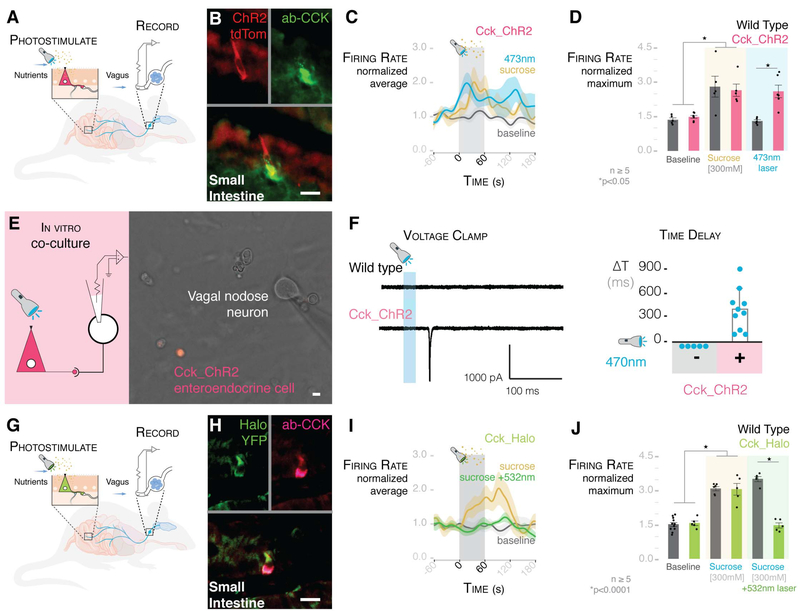
Fig. 4. Millisecond transduction from enteroendocrine cells to vagal neurons.
(A) Model of intraluminal photostimulation and vagal electrophysiology. (B) In CckCRE_ChR2-tdTomato mice, intestinal enteroendocrine cells express ChR2. (C) Normalized traces for 473-nm intraluminal laser, 300 mM sucrose, and baseline in CckCRE_ChR2 mice. Shading indicates SEM. (D) 473-nm intraluminal laser stimulates vagal firing rate in CckCRE_ChR2, but not wild-type, mice (n ≥ 5 mice; *P < 0.05, ANOVA with post hocTukey’s HSD test; error bars indicate SEM). (E) Patch-clamp electrophysiology of neurons (model on left) in coculture with CckCRE_ChR2 cells (image on right). (F) In coculture, 473-nm photostimulation evoked EPSCs (trace on left) in connected nodose neurons (quantification on right) (n = 9 neurons connected to enteroendocrine cells; -, neurons alone; +, neurons cocultured with enteroendocrine cells; ΔT, time between stimulus and onset of EPSCs). Scale of current and time is shown below the trace. (G) Model of intraluminal photoinhibition and vagal electrophysiology. (H) In CckCRE_Halo-YFP mice, intestinal enteroendocrine cells express halorhodopsin (eNpHR3.0). (I) Normalized traces for baseline, 300 mM sucrose, and 300 mM sucrose with 532-nm intraluminal laser. Shading indicates SEM. (J) In CckCRE_Halo, but not wild-type, mice, a 532-nm intraluminal laser abolishes the effect of sucrose on vagal firing rate (n ≥ 5 mice per group; *P <0.0001, ANOVA with post hocTukey’s HSD test; error bars indicate SEM). All scale bars, 10 μm.