Abstract
Background:
Specialized strategies are needed to understand the complex neuropsychological impairments reported in individuals with profound intellectual and multiple disabilities (PIMD) associated with rare genetic disorders.
Methods:
This narrative review focuses on assessment of individuals with Phelan-McDermid Syndrome (PMS) as a condition commonly associated with PIMD. Published case series and prospective studies were reviewed to evaluate approaches to cognitive, language, motor/sensory, and behavioral domains. This review is framed using general principles for neuropsychological evaluation in PIMD.
Results:
Neuropsychological assessment domains and tools varied across published reports. Adaptive behavior measures, out-of-range developmental assessments, and social-communication measures were commonly used. Available findings were used to shape a recommended framework with potential to improve measurement of clinical outcomes and advance scientific discovery.
Conclusions:
The recommended framework outlines an inter-disciplinary and multimodal neuropsychological assessment process relying on modified standardized assessments, functional assessments, and caregiver/informant reports when evaluating individuals with PIMD. Arrested development and skill variability/regression are also discussed as additional, important considerations in neuropsychological evaluation of individuals with PIMD and rare genetic disorders.
Keywords: Intellectual Disability, Phelan-McDermid Syndrome, neuropsychological assessment, cognition
Introduction
Recent estimates indicate that the prevalence of Intellectual Disability (ID) is approximately 10.7/1000 (Maulik, Mascarenhas, Mathers, Dua, & Saxena, 2011). ID is defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; American Psychiatric Association, 2013) by deficits in intellectual functions as assessed by standardized cognitive tests and clinical evaluation, along with deficits in adaptive functioning in at least one area, with classifications of mild, moderate, severe and profound based on support needed. Literature on neuropsychological assessment in ID is largely comprised of the more prevalent subgroups of individuals classified as mild or moderate (Maulik et al., 2011). In contrast, limited efforts have been made to characterize neuropsychological profiles of individuals with profound ID outside of educational and vocational settings (Van der Molen et al., 2010).
Creating psychometrically-sound metrics of neurocognitive and behavioral abilities in individuals with severe to profound ID is a growing priority (Redin et al., 2014; Wright et al., 2015). Reliable and valid neuropsychological evaluation procedures have potential to support clinical care as well as advance research on populations with rare genetic disorders that include a substantial percentage of individuals with severe-to-profound ID. Evaluations in these populations will require specialized attention to measurement challenges, comorbid medical conditions as well as atypical skill progression, including regression. Slowed, variable skill progression and regression during development are particularly challenging trajectories reported in rare genetic conditions (i.e. Rett syndrome). At present, the lack of reliable and valid tools to measure skill variability creates barriers for both clinical care and scientific discovery.
This narrative review seeks to provide an overview of clinical and practical challenges to neuropsychological evaluations of individuals with rare genetic conditions and severe-to-profound ID, and presents a framework to support practitioners who evaluate (and treat) these complex conditions (Simon, Haas-Givler, & Finucane, 2013). We also pose considerations for future directions to guide clinical care and advance scientific discovery of rare genetic disorders associated with severe-to-profound ID in general. Since medical, motoric or sensory impairments are also commonly found in rare genetic conditions associated with severe-to-profound ID, the term profound intellectual and multiple disabilities (PIMD) has been used (Munde & Vlaskamp, 2015)1, and will be employed here. We specify domains that are typically included in a neuropsychological evaluation in PIMD and where evaluation by other specialists are required in the multi-disciplinary care of individuals with rare genetic conditions and PIMD. In addition, although the severity level of ID is now based on adaptive behavior and supports needed, in this review we generally refer to PIMD and other severity level descriptors of ID based on IQ ranges (i.e. to define that severe severity is associated with IQ scores generally between 20–25 to 35–40 and profound severity to IQ scores less than 20–25), consistent with the DSM-IV criteria used when much of this research was conducted (American Psychiatric Association, 2000). Although we use these terms to operationalize severity of ID, we focus below both on measurement of adaptive behavior, utilizing consistent tools with an informant-based approach, as well as the complexities of measurement of IQ in the PIMD population.
We focus here on Phelan-McDermid Syndrome (PMS), one among many medically complicated, rare genetic syndromes, to illustrate a neurodevelopmental condition requiring specialized attention in neurodevelopmental clinics. PMS is rare and associated with deletions and mutations of chromosome 22q13.3 (Phelan et al., 2001), often including SHANK3- a critical gene in the formation, maturation, and maintenance of synapses (Uchino & Waga, 2013), and potentially including many other genes depending upon the deletion sizes (Sarasua et al., 2011). The recent increase in genetic testing and subsequent increased identification and publication of PMS clinical case series provide insights into the severity of delays and impairments associated with the condition. Findings suggest a clinical profile often including moderate to profound ID, arrested or absent speech, autistic-features, and motor impairment (Egger, Zwanenburg, van Ravenswaaij-Arts, Kleefstra, & Verhoeven, 2016; Zwanenburg, Ruiter, van den Heuvel, Flapper, & Van Ravenswaaij-Arts, 2016), with reports of regression across domains (Philippe et al., 2015; Serret et al., 2015) or variability in learning and behavioral presentation at different points throughout the lifespan (Denayer et al., 2012; Sarasua et al., 2014; Vucurovic et al., 2012). Medical features of PMS frequently include varied (usually mild) dysmorphic features, feeding problems, and seizures (Kolevzon, Angarita, et al., 2014; Sarasua et al., 2014). Thus, clinical reports indicate the majority of reported existing PMS cases may be classified as PIMD (Nakken & Vlaskamp, 2007; Soorya et al., 2013).
Review and Framework for Neuropsychological Evaluation of Rare Genetic Disorders associated with PIMD: Example of PMS
Individuals with PMS require evaluation in medical, neurological, behavioral, and cognitive domains, which are inherently complex in rare genetic conditions associated with PIMD. This review seeks to provide a summary of data, as well as guidance on selection, administration, and interpretation of assessment strategies to support practitioners helping families manage PIMD in the context specific rare conditions. While PIMD may be associated with neurologic conditions arising from environmental factors as well as rare genetic conditions (Mahone & Slomine, 2008), the focus of this review is on associations with rare inherited and de novo genetic conditions.
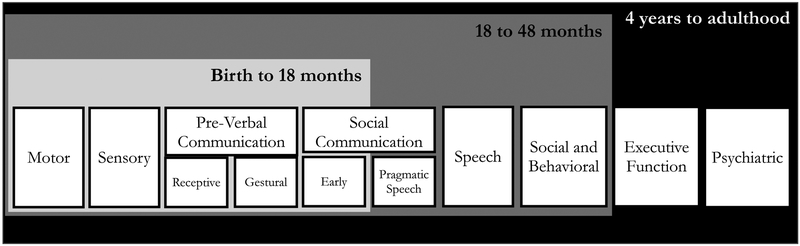
The framework for this review, and recommendations which follow, draws from recognized approaches for general developmental neuropsychological assessment (Holmes-Bernstein & Waber, 1990; Mahone & Slomine, 2008) as well as specific recommendations for individuals who are minimally verbal (Kasari, Brady, Lord, & Tager-Flusberg, 2013). These best practices involve use of multi-method, multi-informant, caregiver- and clinician-administered assessments – an essential strategy for clinical conditions where standardization parameters are less reliable and feasible. Since individuals with PIMD associated with rare genetic conditions often display motor and other multi-system sensory impairments, we indicate domains that require evaluation beyond that provided by a neuropsychologist. Neuropsychological evaluations in PMS and similar conditions should be preceded by thorough medical/neurological examination (Kolevzon, Angarita, et al., 2014). Traditional neuropsychological assessment domains such as cognition and adaptive functioning, language skills, and psychiatric comorbidities including autism spectrum disorder (ASD), mood, and behavioral disturbance are also outlined in the framework (Figure 1).
Figure 1.
Conceptual Framework for Decision Points of Assessments
Several considerations, including some that break from traditional neuropsychological assessment guidelines, are recommended for individuals with PIMD and rare genetic conditions. In addition to multi-method and interdisciplinary evaluations, assessment strategies for cognitive and intellectual functioning, the cardinal feature of PIMD, often require extensive modifications. Importantly, this review highlights the limits of standardized neuropsychological assessments in certain domains, for certain developmental levels, and emphasizes the use of tools to inform descriptive profiles of strengths and weaknesses (Tenorio, Campos, & Karmiloff-Smith, 2014). Adaptations often involve changes to standardization procedures, alternate scoring methods (e.g. use of raw scores or age equivalents), and use of measures normed in chronologically younger populations. Structured clinical observations, parent/caregiver reports, and functional assessments also contribute to systematic and clinically meaningful evaluations of other domains. While many of these principles are applicable to pediatric neuropsychology in general, this broad assessment approach is particularly relevant for individuals with PIMD and rare genetic conditions.
General Principles for Neuropsychological Evaluation in PIMD:
The use of a multi-informant and multi-assessment caregiver- and clinician-administered battery is necessary given the limitations of available standardized instruments and direct assessment methods.
Assessment of motor, medical, and psychiatric comorbidities prior to specific neuropsychological evaluation components is needed to determine the extent to which physical and/or sensory impairments may limit choice of tests and/or interpretation of results. Basic fine motor abilities, such as pointing, are critical when selecting tests for domains such as memory, visual-spatial skills and even receptive vocabulary. Motor limitations may necessitate consideration of non-traditional assessment strategies and technologies that minimize motor and speech domains such as those employed in populations of individuals with cerebral palsy (Warschausky et al., 2012).
Use of unstandardized approaches to estimate an individual’s intellectual functioning is necessary. Administration of direct developmental tests (when floor effects are present with chronological age-normed standardized IQ tests) is strongly recommended and informative. Though imperfect, raw scores or mental age (MA) estimates are the best (and only) available indicators of when out-of-age-range developmental tests are used. Age equivalent scores are readily available from developmental tests, as well as from many adaptive behavior measures. Most measures of adaptive behavior are developed and standardized with a lifespan scoring system allowing evaluators to obtain raw scores, age equivalents, and standardized scores.
A child should not be deemed “untestable” due to the lack of available standardized measures for an individual with PIMD. Behavioral and educational strategies will need to be used if a child is uncooperative after repeated attempts. These may include engaging the caregiver to help guide effective strategies used at home/school (e.g. sensory breaks) or motivators that may be used or saved for testing.
Developmental Considerations in Domain and Test Selection (Figure 1)
MA is estimated to be <18 months (approximately). Developmentally, infants up to approximately 18 months old are just starting to talk and develop basic executive functioning skills (Diamond, 2013). Relevant assessment domains at this development level include: motor and sensory, developmental functioning (as a proxy for IQ), adaptive behavior, pre-verbal communication (receptive, gestural), early social communication skills, and behavior. Most standardized IQ tests will have insufficient sampling and standardization in individuals functioning at the lower poles of standardization distributions. As such, standardized scores are of limited value in PIMD associated with rare genetic conditions. Alternate measures and score reporting (e.g. age equivalents) will need to be considered. Measurement of social-communication will also require an alternative approach since available standardized measures, such as the Autism Diagnostic Observation Schedule-2 (Lord et al., 2012), have reduced sensitivity and specificity in individuals functioning below a developmental level of 15–18 months (Risi et al., 2006). Standardized measures of attention and executive functions are less likely to be utilized in PIMD evaluations as existing standardized measures do not capture these constructs in early stages of development (i.e. under 18–24 months). Still, behavioral measures are an important component of the neuropsychological evaluation in all individuals with PIMD. Individuals functioning at this level will benefit from functional behavioral assessment (FBA) and related behavioral analytic approaches. Systematic clinical observation and parent-caregiver report should be weighed heavily.
MA: 18–48 months (approximately). Proceed with broad evaluation of social-communication, speech, social and behavioral measures and utilize behavioral/functional tests described above as needed. The delineation of skills at approximately an 18 month level presumes sufficient verbal and social-communication skills to respond to standardized assessment of some neurodevelopmental disorder diagnoses (e.g. Autism Spectrum Disorder) but likely not others (e.g. Language Disorder, Social Communication Disorder).
MA >48 months (which, depending on other factors, may not be considered PIMD). Additional domains including psychiatric symptoms and executive functions and areas such as language and memory may be reliably assessed, though adaptations may be needed to interpret out of range testing.
Review & recommendations for neuropsychological assessment domains in PMS
Sixteen published case studies and prospective cohort studies of individuals with PMS were reviewed and are summarized below by assessment domain. Summaries are provided in tables and text with the aim of highlighting strengths and weaknesses of measures and techniques, and make suggestions for selection of a comprehensive testing battery for individuals with PMS, which we propose may be extended to the greater PIMD field.
Motor & Sensory
Motor and sensory domains are important foci in the evaluation of individuals with rare genetic conditions associated with PIMD, and although additional evaluations by specialists (e.g. ophthalmologists, audiologists, physiatrists and neurologists) are required for specification of these areas, neuropsychological evaluations can include some assessment of these domains. In addition to requisite screening and history taking procedures as part of standard neuropsychological exams, evaluations in rare genetic conditions associated with PIMD must also evaluate delays, specific motor challenges, as well as functional impairments in these areas. In PMS, early and persistent hypotonia leading to gross and fine motor delays have been reported consistently, but very little additional description has been provided. Other neurodevelopmental conditions associated with PMID (Nakken & Vlaskamp, 2007) also exhibit physical, motor impairments, with examples including gait abnormalities in Rett Syndrome (Gadalla, Ross, Riddell, Bailey, & Cobb, 2014) and ataxia in Fragile X Syndrome (Grigsby et al., 2008).
Motor impairments in PMS have been predominately reported through clinical neurological evaluations. Direct, standardized measures are less frequently used and are constrained in ways similar to standardized cognitive and language tests. Motor tests used in published reports of PMS include the Peabody Developmental Motor Scale (Denayer et al., 2012), which assesses a range of motor skills in young children (birth to 60 months) and has domains assessing reflexes, stationary, locomotion, object manipulation, grasping, and visual-motor integration, with composite scores for total, fine, and gross motor. Additionally, the Vineland Adaptive Behavior Scales motor domain subscales (Soorya et al., 2013; Sparrow, Cicchetti, & Balla, 2005) and the Bayley Scales of Infant & Toddler Development-III (Bayley & Reuner, 2006; Zwanenburg et al., 2016) have been used to assess motor skill development.
In the sensory domain, individuals with PIMD may present with basic sensory deficits, including but not limited to ophthalmologic abnormalities such as cortical visual impairment (Bosch et al., 2014), strabismus and esotropia (Jeffries et al., 2005; Sarasua et al., 2014). In PMS, both vision and hearing impairments, as well as more general sensory sensitivity differences are commonly reported (Kolevzon, Angarita, et al., 2014) and may mirror basic science findings such as photosensitivity, decreased pain sensitivity and aversions, all reported in mouse models (Han et al., 2016; Kouser et al., 2013; Peca et al., 2011).
In addition, sensory hyper- and hypo-reactivity are ubiquitous but generally nondescript features in PIMD and other neurodevelopmental conditions. Excessive chewing of non-food objects (i.e. pica) and abnormal reactions to changes in temperature and touch have been reported in individuals with PMS (Phelan et al., 2001; Sarasua et al., 2014). A recent study comparing sensory behaviors in children with PMS and idiopathic ASD found that approximately 80% of respondents in both groups exhibited atypical sensory responses on the Short Sensory Profile relative to the standardization sample (Mieses et al., 2016). While intriguing, several design limitations limit interpretation, including a significant cognitive functioning difference between samples. In addition, the use of the Short Sensory Profile, a measure normed in typically developing children above 3 years, limits interpretation of disease-specific versus developmentally typical sensory behaviors in individuals with PMS, and others with PIMD.
Selecting motor & sensory measures
Table 4 summarizes guidelines for selecting neuropsychological domains and tools for evaluation of PIMD and PMS. In addition to evaluations required by other specialists, features such as persistent hypotonia (Phelan et al., 2001) and functional skill impairments related to motor functioning are important to consider during neuropsychological evaluations. The literature review and results in Table 4 underline the lack of direct, standardized measures available to quantify motor and sensory impairments in PMS and PIMD more generally. To date, the majority of published reports rely on caregiver report and careful clinical evaluation to characterize motor and sensory functioning in PMS. Qualitative data gathered from out-of-age-range motor domains on developmental measures can provide useful clinical information. The Vineland-3 motor domains, including out-of-range administration of the gross motor domain, are also valuable clinical evaluation tools.
Table 4.
Guidelines for selecting neuropsychological domains and tools for evaluation of PIMD
| Assessment domain | Methods | Sources | Considerations for test administration & interpretation |
|---|---|---|---|
| Motor and Sensory | Medical record review | Developmental motor history, neurological exams, physical exams, and/or physical therapy evaluations. | Clinical assessment from medical subspecialists and allied health professionals should be incorporated |
| Caregiver/parent report of motor abilities | Motor subscales of adaptive behavior measures Motor subscales of developmental assessments |
Scales such as the Vineland-III, MSEL, and ABLLS-R may be informative | |
| Caregiver/parent report of sensory abilities | Clinical interviewing Infant/Toddler versions of standardized assessments (e.g. Sensory Profile) |
Many standardized measures of sensory impairment are normed in individuals with mental and chronological ages above 36 months | |
| Intellectual Functioning | Direct assessment | Developmental assessments | Useful in PIMD individuals with MA <48 months and limited verbal or motor abilities. Use mental AE or raw score metrics for reporting and tracking. |
| Non-traditional IQ tests | Tests such as the Differential Abilities Scale –II include standard score floors as low as 25, extended norms for older individuals requiring preschool version (Elliott, 2007) | ||
| Adaptive behaviors | Parent/caregiver report | Standardized parent-caregiver report | Most standardized adaptive behavior measures lack standardization in profound range of functioning (<20) |
| language and Communication | Observation in natural settings | Language sampling (e.g. mean length of utterance, inventory of speech sounds) | Consider using mean length utterance (MLU) or diary/inventory of speech sounds to estimate expressive language Caregiver report forms of spoken sounds and words designed for infants and toddlers such as the MacArthur Bates Communication Development Inventory (MBCDI) |
| Behavior data recording | Functional communication skills | Observation in FBA or direct assessment in curriculum-based assessments such as the ABLLS-R | |
| Direct assessment & caregiver report | Receptive language | Motoric limitations may impact ability to assess basic receptive vocabulary (Brady, et al, 2014) | |
| Direct assessment & Observation | Pre-linguistic communication | Evaluate joint attention, reciprocal imitation, symbolic play, and gestural communication (e.g. Early Social Communication Scale) | |
| Social-communication/ASD | Direct assessment | Standardized semi-structured interview with child | Standardized measures such as the ADOS-2 are valid in individuals with MA>12–15 months; can use presses from standardized measures to inform clinical observations (see prelingustic communication above) |
| Parent/caregiver report | Standardized interview with parent/caregiver | Standardized measures such as the ADI-R are valid in individuals with MA>18–24 months; can use adapted interview to inform diagnostic formulation | |
| Behavior/psychiatric | Observation Parent/caregiver report |
Standardized parent-caregiver report Clinical behavioral assessment |
Some standardized measures are developed for or tested in individuals with ID. Functional behavioral assessment (FBA) are also recommended to inform diagnosis and treatment planning |
ABLLS-R-assessment of basic language and learning skills, revised
ADI-R – Autism Diagnostic Interview, Revised
ADOS-2 = Autism Diagnostic Observation Scale, Second Edition
AE = age equivalent
ASD = autism spectrum disorder
ID = intellectual disability
IQ = intellectual quotient
MA = mental age
MSEL = Mullen Scales of Early Learning
PIMD = profound intellectual and multiple disabilities
Several efforts are underway to develop direct standardized measures of sensory hyper-and hypo-sensitivity in neurodevelopmental disabilities (Siper, Kolevzon, Wang, Buxbaum, & Tavassoli, 2017). In the meantime, parent reports during diagnostic interviews such as the Autism Diagnostic Interview-Revised (ADI-R), clinical observations, and standardized caregiver reports anchor clinical assessment of sensory issues in PIMD and rare genetic disorders.
Intellectual Functioning
SHANK3 gene disruption is reported to be highly penetrant and strongly associated with ID, with one study showing SHANK3 deletions and mutations present in 1.7% of those with intellectual disability (Gong et al., 2012). Indeed, several studies have reported descriptions of PIMD in PMS. Table 1 includes findings from five prospective cohort studies (Jeffries et al., 2005; Phelan et al., 2001; Philippe et al., 2008; Soorya et al., 2013; Zwanenburg et al., 2016) as well as other reports. Using age equivalents (AE), MA scores fall within the 18–36 month range across domains across a wide range of chronological ages (Denayer et al., 2012; Philippe et al., 2008; Soorya et al., 2013; Zwanenburg et al., 2016), underscoring the high prevalence of profound ID in PMS. In a prospective study of 32 individuals with PMS ages 1.6 to 45.4 (mean age = 8.8± 9.2 years), the maximum AE achieved on standardized cognitive measures was 33 months, in a 53 month old participant (Soorya et al., 2013). Zwanenburg, et al (2016) studied a pediatric cohort (median age: 56 months) and reported a mean age equivalent under 18 months. Further, the maximum age equivalent reported in a subsample of older children with chronological age over 9 years old was 34 months (Zwanenburg et al., 2016).
Table 1.
Cognitive and developmental assessments reported in PMS studies
| Measure | Standardized Age Range | Administration Method | Description and Domains | PMS Studies: Cohort Descriptions | PMS Studies: Average Reported Mental Age Equivalent |
|---|---|---|---|---|---|
| Bayley Scale of Infant and Toddler Development- Third Edition (BSID-III) (Albers & Grieve, 2007) | 1–42 mos. | Direct testing, with parent/caregiver input; 60 minutes |
Cognitive, Language, Motor, Social/emotional, Adaptive behavior | n=4; age range: 23 mos-34 yr.; (Denayer et al., 2012) | 10.4 mos. |
| n=32; age range: 8–178 mos.; (Zwanenburg et al., 2016) | 15.6 mos. | ||||
| Snijders-Oomen Nonverbal Intelligence Test (SON) (Snijders et al., 1989) | 2.5–17 yrs. | Direct testing by clinician; 50 minutes | Nonverbal: Spatial, Insight, Reasoning abilities | n=1; age: 108 mos.; (Denayer et al., 2012) |
36–42 mos. |
| Leiter International Performance Scale-Revised (LIPS) (Roid & Miller, 2011) |
3–75 yrs. | Direct testing by clinician; 20–45 minutes |
Nonverbal: Reasoning, Visualization, Memory, Attention | n=1; age: 59 mos.; (Simenson, Oiglane-Shlik, Teek, Kuuse, & Ounap, 2014) |
41 mos. |
| n=2; (Soorya et al., 2013) | AE N/A | ||||
| n=2; age range: 71–109 mos.; (Kolevzon, Bush, et al., 2014) | 33.5 mos. | ||||
| Wechsler Intelligence Scale for Children (WISC-III) (Wechsler, 1991) | 6–16 yrs. | Direct testing; 48–65 minutes |
Verbal Comprehension, Freedom from distractibility, perceptual organization, Processing speed | (n=1) (Simenson et al., 2014) | AE N/A |
| n=1; age: 104 mos.; (Philippe et al., 2008) |
IQ: 51 | ||||
| Wechsler Preschool and Primary Scales of Intelligence, Third Edition (WPPSI-III) (Wechsler, 2002) |
2:6–7:7 yrs. | Clinician administered & rated; 45–60 minutes |
Verbal, Fluid reasoning, Working memory, Processing speed | n=1; age: 119 mos.; (Zwanenburg et al., 2016) | 52 mos. |
| Griffiths Mental Developmental Scale (GMDS) (Griffiths, 1976) | Birth-8 yrs. | Direct testing by clinician; 50–60 minutes |
Development in: Locomotor, Personal-social, Hearing & language, Eye and hand coordination, Performance, Practical reasoning |
(n=1) (Simenson et al., 2014) | AE N/A |
| Stanford Binet Intelligence Scale- Fifth Edition (SB-5) (Roid, 2003) | 2–85 yrs. | Direct testing by clinician; 50 minutes |
Fluid reasoning, Knowledge, Quantitative reasoning, Visual-spatial processing, Working memory IQ scales: Verbal, Nonverbal, Full |
(n=1) (Soorya et al., 2013) | AE N/A |
| Parental Involvement Project (PIP) Developmental Charts (Jeffree & McConkey, 1998) | Birth-60 mos. | Parent/Caregiver rated | Gross motor, Fine motor, Self-care, Play, Language | (n=35) (Jeffries et al., 2005) | AE N/A |
| Battelle Developmental, Inventory (BDI) (Newborg, Stock, Wnek, Guidubaldi, & Svinicki, 1988) | Birth-7 yrs. | Direct testing by clinician; 90 minutes |
Personal-social, Adaptive, Motor, Communication, Cognitive | n= 20; age range: 2–26 years; (Phelan et al., 2001) |
Cognitive AE: 9.3 mos. Motor AE: 11.3 mos. |
| Mullen Scales of Early Learning (MSEL) (Mullen, 1995) | Birth-68 mos. | Direct testing by clinician; 40–60 minutes |
Gross motor, Visual perception, Fine motor, Expressive language, Receptive language | n= 27; age range: 1.6–45 years; (Soorya et al., 2013) |
Receptive AE: 11.63 mos. Expressive AE: 7.52 mos. Fine Motor AE: 14.89 mos. Gross Motor AE: 17.95 mos. |
| n=24; age range: 2–11 years; (Mieses et al., 2016) | Mean DQ: 29.2 | ||||
| n=7; age range: 61–177 mos.; (Kolevzon, Bush, et al., 2014) | 12.7 mos. | ||||
| Psychoeducational, Profile Revised (PEP-R) (E Schopler et al., 1990) | 6 mos. - 7 yrs. (functional level) 6 mos. - 12 yrs. (chronological age) |
Direct testing by clinician; 45–90 minutes |
Imitation, Perception, Fine motor, Gross motor, Hand-eye integration, Cognitive performance, Cognitive verbal; Behavioral assessment: Play, Affect, Interest in materials, Sensory, Language | n=7; age range: 51–136 mos.;(Philippe et al., 2008) |
Cognitive performance AE: 23.7 mos. Cognitive Verbal AE: 15 mos. |
AE = age equivalent
DQ = developmental quotient
IQ = intellectual quotient
Mos. = months
N/A = not available or not reported
PMS = Phelan-McDermid Syndrome
Yrs. = years
These findings should be considered in the context of known challenges in using standardized cognitive tests in individuals with PIMD, most notably the presence of floor effects. Floor effects result from several factors including inadequate population sampling in the 55–70 range of IQ, and almost no sampling in the IQ range < 55. Also, standard scores (i.e. deviation IQ scores) are determined largely through extrapolation in the lower poles of the standard distribution (Whitaker, 2008). A limited number of items assessing early learning and cognition also contribute to floor effects. By DSM-IV and many research definitions, profound ID is associated with scores in the 20’s or below. Thus, traditional standardized IQ tests, albeit with some exceptions such as the Differential Ability Scales, 2nd edition (Elliott, 2007) have floors that often do not go lower than 40 (mean = 100, standard deviation = 15) and do not provide adequate range to provide standardized scores in PIMD.
In response to challenges with standard score calculation and interpretation in PMS studies, we report MA scores in Table 1. Noting that the selection of alternative scores as a proxy for IQ is not straightforward, MA estimates from age equivalent scores provide several advantages over deviation IQs (which are often not even possible to obtain) for tracking and monitoring in rare genetic disorders such as PMS. MA is calculated by comparing an individual’s raw score to the corresponding age equivalent. Though criticized when used as an IQ alternative in the general population, MA is likely more accurate than deviation IQ in PIMD populations since true versus extrapolated scores are used. Compared to deviation IQ, MA is not relational (i.e. 18-month developmental age is the same regardless of chronological age) and therefore, more suitable for parametric analyses in research (Whittaker, 2008). Limitations of MA estimates include: (1) MA is averaged for a given age group and requires treatment as an ordinal variable, and (2) MA plateaus based on test used and skill level. Thus, while MA estimates are useful for population level assessment relative to deviation IQs, using MA to measure change across time presents interpretation challenges.
Ratio IQ scores (mental age/chronological age X 100) are commonly used alternatives to MA but present concerns related to over- or under-estimating scores. The underlying assumption of ratio IQ scores presumes that intelligence proceeds in linear trajectories throughout development. This assumption is inconsistent with known patterns of discontinuous growth in cognitive and social skills in typical and atypical development. Ratio IQs are particularly problematic in assessing and predicting IQs in older ages when intelligence plateaus (Weintraub, Dikmen, et al., 2013) and therefore likely to depict declining scores as an individual ages (Bishop, Farmer, & Thurm, 2014).
Selecting intellectual functioning measures (or proxies)
Tests reported in Table 1 also highlight the reliance on non-traditional measures for estimating cognition in PIMD evaluations. Measures include: 1) developmental/infant cognition measures such as the Bayley Scales of Infant and Toddler Development:Bayley-III (Bayley & Reuner, 2006) and Mullen Scales of Early Learning (MSEL; Mullen, 1995; Soorya et al., 2013; Zwanenburg et al., 2016), which provides a score for visual reception 2) nonverbal intelligence scales that do not require any functional speech such as the Leiter International Performance Scales (Roid & Miller, 2011) and Snijders-Oomen Nonverbal Intelligence Scale (Denayer et al., 2012; Snijders, Tellegen, & Laros, 1989); and 3) educational tests such as the Psychoeducational Profile Revised (PEP-R; Philippe et al., 2008; Schopler, Reichler, Bashford, Lansing, & Marcus, 1990).
Overall, direct cognitive assessment using out-of-range measures, raw scores, and developmental or MA equivalence scores provide the best available practice in evaluations of PMS. However, several limitations require consideration when utilizing out-of-range or alternative cognitive measures in PIMD (Table 4). First, nonverbal IQ may have limitations in PIMD if an individual does not possess the prerequisite skills in 2-D visual discrimination, visual scanning of multiple items. Nonverbal IQ tests as well as items on developmental tests such as the MSEL may also be confounded by motor impairments. Even tests appropriate for minimally verbal individuals require a proximal point, or ability to move a small card. Tests such as the MSEL include motor scales in calculations of summary scores, which may confound and deflate scores. Finally, although advantages of MAs calculated from age equivalents are discussed above, tests vary in methods for calculation of age equivalent scores. Specifically, the MSEL provides age equivalents in small, monthly bands. However, other tests such as the DAS-II include larger bands (3 months or more) for age equivalents, and do not differentiate age equivalents below approximately 2 and a half years.
Adaptive Behavior
Adaptive behavior profiles are independent of IQ but integral to diagnosis and severity specification of ID (Bertelli et al., 2014). Deficits in adaptive behavior, or the actual ability to carry out tasks of daily living, have been a criterion for a diagnosis of ID since the publication of the Diagnostic and Statistical Manual of Mental Disorders - Third Edition (DSM-III) (American PsychiatricAmerican Psychiatric Association, 1980), in part due to the limitations of cognitive measures discussed above. As noted, the DSM-5 now emphasizes the role of adaptive functioning impairments in conceptual, social and practical domains within the diagnosis of ID in part by defining severity levels based on supports needed for adaptive skills (American Psychiatric Association, 2013).
To illustrate impairments in functional skills attainment in conditions associated with PIMD such as PMS, we describe a study reporting considerable disparity between chronological age and adaptive functioning level in individuals with PMS. Phelan, et al. (2001) reported on Vineland Adaptive Behavior Scales, Second Edition (Phelan et al., 2001; Sparrow et al., 2005) in 20 individuals with PMS, ranging from 24 months to 26 years, with a mean age of 7 years. While standard scores means ranged from 35 to 45, mean age equivalent scores give a greater sense of degree of impairment, since most children were past the preschool chronological range. These ranged between 11.4 months (communication domain) and 14.7 months (daily living domain). Other studies, which mainly included school-age children to adult subjects, found similar disparities between chronological age and adaptive functioning level, with average age equivalents ranging from the 10 month level to the 20–40 month level with age equivalents at the higher end of this range most commonly in adults (Serret et al., 2015; Soorya et al., 2013; Verhoeven, Egger, Willemsen, de Leijer, & Kleefstra, 2012).
Selecting adaptive behavior measures
Measurement of adaptive behavior traditionally involves caregiver report of an individual’s actual performance (versus perceived ability to carry out tasks) with common scales including the Vineland Adaptive Behavior Scales, Third Edition (Vineland-3; Sparrow, Cicchetti, & Saulnier, 2016), the Adaptive Behavior Assessment Scales, Third Edition (ABAS-3; Harrison & Oakland, 2015), the Vineland Screener (Sparrow, Carter, & Cicchetti, 1993), and Scales of Independent Behavior-Revised (SIB-R; Bruininks, Woodcock, Weatherman, & Hill, 1996).
The Vineland-3, ABAS-3, and SIB-R are psychometrically sound and clinically useful measures of adaptive behavior in clinical practice and have several advantages over IQ tests. As previously mentioned, caregiver reports provide valuable clinical information on difficult to assess domains such as motor ability and functioning. The measurement of multiple domains in a single instrument and standardization from birth to adulthood allows for age equivalents and profile analyses of differentials in various areas (e.g. socialization versus motor functioning).
The Vineland is often used in research studies of other rare genetic conditions. The availability of published, disease-specific data has appeal in providing comparisons for evaluating individual-level patient progress. The availability of growth scale values (GSVs) in the Vineland-3 (Sparrow et al., 2016) is also a notable advantage, as GSV scores allow for tracking individual growth in each domain. Although the Vineland-3 has more items in certain domains at the lower developmental levels, standard scores continue to exclude a category of profound deficit (<20).
Expressive and Receptive Language and Communication
By definition, most individuals with severe-to-profound ID have limited expressive language. With respect to PMS, cohort studies have indicated very low verbal mental ages (Soorya et al., 2013; Zwanenburg et al., 2016). In addition, a published report from 50 cases with questionnaire-based language data found 78% had fewer than 40 words and 22 % used fluent phrases (Sarasua et al., 2011).
Developmental assessment and non-traditional language assessment are frequently used to evaluate language in individuals with PMS. Published studies have used direct measures, e.g. the Non-Speech Test (Zink & Lembrechts, 2000), Reynell Developmental Language Scale (Reynell & Gruber, 1984); and parent/caregiver report measures, e.g. McArthur-Bates Communicative Development Inventory (Fenson et al., 2007), communication domain of the Vineland (Sparrow et al., 2005). However, even these measures may exhibit floor effects. Denayer, et al. (2012) found that only 3 of 7 study participants could achieve scores on language testing of either the Non-Speech Test or the Reynell Developmental Language Scale. To date, commonly used receptive language measures such as the Peabody Picture Vocabulary Test (PPVT; Dunn & Dunn, 2007) have not been reported to be successfully administered, likely due to challenges with motor planning, attention, visual scanning and/or pointing (Brady, Anderson, Hahn, Obermeier, & Kapa, 2014).
Selecting expressive and receptive language & communication measures
While there is a plethora of measures available to assess early language skills in individuals with some verbal abilities (see Tager-Flusberg et al., 2009), assessment in PIMD populations with limited language and motor skills requires integration of observation, direct assessment, and parent report of broader communication skills across domains. Table 4 provides an overview of strategies and categories for assessing language in PIMD. Use of language sampling (Kasari, et al, 2013) to calculate measures such as mean length of utterance (MLU) is strongly recommended within the clinical session as well as reports from home. Evaluation of receptive language abilities through developmental and adaptive behavior measures is also recommended. Finally, observation of pre-linguistic communication skills such as attention to speech sounds and gestures helps inform potential early social communication impairments. Observation of augmentative and alternative communication (AAC) devices should occur when appropriate during the evaluation with evaluators “testing the limits” of vocabulary and communication bids. In addition, referrals for AAC evaluations should be considered in many cases.
Autism symptom domains
PMS is one of several genetic disorders associated with ID that has biological and behavioral overlap with ASD. SHANK3 deletions, but primarily mutations, have also been identified in approximately 0.5% of individuals with ASD, shown in a review of combined samples (Betancur & Buxbaum, 2013) and up to 2% in cases of ASD associated with moderate to profound ID (Leblond et al., 2014). As such, PMS has garnered substantial interest as a genetic condition with potential to inform etiological and treatment research in ID and ASD. Heightened symptoms and diagnoses of ASD have been reported in individuals with PMS with rates between 60–90% depending on methods and samples (Phelan, et al., 2001, Jeffries, et al, 2005, Dhar, et al, 2010, Soorya, et al, 2013).
Clarifying ASD symptom profiles in PMS reflects a diagnostic challenge faced across many neurodevelopmental conditions. Namely, the specificity of most ASD diagnostic tools is reduced with the severity of ID (Risi et al., 2006), and with conditions with comorbid sensory or motor impairments (Sappok et al., 2013; Thurm et al., 2016). Specifically, the Autism Diagnostic Observation Schedule–2nd edition (ADOS-2) and the Autism Diagnostic Interview-Revised (ADI-R) have consistently shown very low specificity when used with individuals with profound ID based on MAs at or below 18 months (Risi et al., 2006) and are restricted to individuals with at least a 12 month (ADOS-2) or 18 months to 2 year (ADI-R) MA (Lord et al., 2012; Rutter, LeCouteur, & Lord, 2003). The relationship between developmental level and both ADOS-2 and ADI-R scores may also vary by symptom domain. When controlling for expressive language, non-verbal IQ, and ADOS Module, a weaker association is found between developmental level and repetitive/restricted behaviors compared to social-communication symptoms on the ADI-R (Lord, Rutter, & Le Couteur, 1994).
The conflation between severity of ID and ASD symptoms is of most concern for genetic disorders associated with PIMD (Moss & Howlin, 2009). Table 2 presents the studies of PMS that have used standardized autism diagnostic measures, and illustrates the variable, but generally high rate of meeting cutoffs on these instruments. Importantly, a recent study of PMS found that the degree of developmental delay predicted which individuals received an ASD diagnosis (Oberman, Boccuto, Cascio, Sarasua, & Kaufmann, 2015).
Table 2.
Social-communication assessments reported in PMS studies
| Measure | Standardized Age Range | Administration Method | Description and Domains | PMS Studies: Cohort Descriptions & Clinically Relevant Findings |
|---|---|---|---|---|
| Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 2012) | >12 mos. | Direct testing by clinician; 40–60 minutes |
Toddler Module Module 1: pre-verbal/single words Module 2: phrased speech Module 3: complex speech Module 4: complex speech, late teens and adults. |
Scores N/A (n=1) (Dhar et al., 2010) |
|
Subjects meeting autism criteria: 23 Subjects meeting ASD criteria: 4 (n=32) (Soorya et al., 2013) | ||||
| Scores N/A.(n=2) (Serret et al., 2015) | ||||
| Scores N/A. (n=9) (Kolevzon, Bush, et al., 2014) | ||||
| Scores N/A. (n=24) (Mieses et al., 2016) | ||||
| Autism Diagnostic Interview Revised (ADI-R; Rutter, LeCouteur & Lord, 2003) | >2 yrs. | Parent/caregiver interview by clinician; 90–150 minutes |
Communication Reciprocal Social Interactions Repetitive Behaviors/Interests |
Scores N/A. (n=1) (Dhar et al., 2010) |
| Subjects meeting ASD criteria: 18 (n=30) (Soorya et al., 2013) | ||||
| Subjects meeting ASD criteria: 0 (n=8) (Philippe et al., 2008) | ||||
| Scores N/A. (n=2) (Serret et al., 2015) | ||||
| Scores N/A. (n=9) (Kolevzon, Bush, et al., 2014) | ||||
| ADI-R algorithm scores N/A (n=40) (Oberman et al., 2015) | ||||
| Gilliam Autism Rating Scale 3 (GARS-3) (Gilliam, 1995) | 3 – 22 yrs. | Parent or Teacher rated; 5–10 minutes | DSM-5 Autism Spectrum Disorder | Scores N/A. (n=1) (Dhar et al., 2010) |
| Childhood Autism Rating Scale (CARS) (Eric Schopler, Reichler, & Renner, 2002) | 2+ yrs. | Clinician rated; 5–10 minutes | Autism diagnosis | Scores N/A. (n=1) (Dhar et al., 2010) |
|
Subjects above ASD cutoff score: 17 (n=18) (Phelan et al., 2001) | ||||
|
Subjects above ASD cutoff score: 2 (n=2) (Serret et al., 2015) | ||||
| Social, Communication Questionnaire (SCQ) (Berument, Rutter, Lord, Pickles, & Bailey, 1999) | >4 yrs. (mental age: >2 yrs.) |
Parent/caregiver rated; 10 minutes |
Autism symptoms |
Subjects above ASD cutoff score: 23 (n=27) (Jeffries et al., 2005) |
| Pervasive Developmental, disorder in Mentally Retarded Persons (PDD-MRS) (Kraijer & de Bildt, 2005) | 2–55 yrs. | Parent/caregiver rated; 5–10 minutes |
Autism Risk |
Subjects scoring within PDD spectrum: 4 (n=7) (Denayer et al., 2012) |
AE = age equivalent
ASD = autism spectrum disorder
DSM-5 = Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
Mos. = months
N/A = not available or not reported
PDD = pervasive developmental disorder
PMS = Phelan-McDermid Syndrome
Yrs. = years
In sum, categorical assessment of ASD is challenging in individuals with PMS and those with PIMD (Harris, 2016). Revisions to ASD criteria in the DSM-5 begin to address the challenge through the addition of Criterion E, which requires consideration that social communication deficits (criterion A) and restricted and repetitive behaviors (criterion B) are “not better explained by intellectual disability or global developmental delay.” However, Criterion E has not been operationalized to date. The complexity of the diagnostic overlap between ASD and PMID (Bodfish, Symons, Parker, & Lewis, 2000; Havdahl et al., 2016) may suggest a need for different diagnostic thresholds (compared to thresholds set from published diagnostic measure algorithms, for instance) to inform research on genetic conditions associated with PIMD and ASD.
Selecting autism symptom measures
Table 4 reviews available strategies for social-communication symptoms and ASD assessment and provides guidance for use in individuals outside of standardization range. Early social-communication behaviors such as gestures, speech sounds, and functional communication are not reliably assessed with available standardized tools in individuals with rare genetic disorders and PIMD. Adaptations of measures such as the ADOS-2 for older, nonverbal individuals now exist, and these may also inform efforts to disentangle ASD and PIMD symptom profiles (Hus et al., 2011). However, even these adaptations are not developed for scoring to be discriminatory when MAs are below 18 months. Knowledge about ASD symptoms in very young children may support research on the reliable assessment of social-communication and repetitive behaviors in populations with very low MAs, although there are qualitative differences in older individuals with MAs below 18 months, compared to toddlers. For example, preferential attention to communicative speech has been shown to be a critical early precursor of social communication skills, in young children at risk for ASD (Osterling & Dawson, 1994) so may be useful to look at.
Behavioral and Psychiatric Domain
Behavioral and psychiatric symptoms in medically complex conditions such as PMS include mouthing/pica, eloping, social anxiety/withdrawal, and specific aggressive behaviors, including hair pulling (Sarasua et al., 2014; Verhoeven et al., 2012). Reports of behavioral changes during the life course and diagnosis of psychiatric conditions such as bipolar disorder and schizophrenia in PMS also support the need for detailed psychiatric evaluations (Denayer et al., 2012; Messias, Kaley, & McKelvey, 2013; Verhoeven, Egger, Cohen-Snuijf, Kant, & de Leeuw, 2013; Verhoeven et al., 2012). Indeed, several genes on chromosome 22q13 are implicated in the pathogenesis of schizophrenia and bipolar disorder (Liang et al., 2002; Verma, Kubendran, Das, Jain, & Brahmachari, 2005). Sleep problems are also commonly reported in PMS and have been associated with challenging behavior in PIMD (Poppes, van der Putten, Post, & Vlaskamp, 2016).
Shaw, et al. (2011) conducted the most thorough evaluation of behavioral and psychiatric profiles in individuals with PMS in the published literature. Results suggested elevated rates of attention deficit/hyperactivity disorder (ADHD), bipolar, and psychotic symptoms in individuals with PMS. However, the authors report several challenges in interpreting data as indication of psychiatric conditions in PMS. Elevated mood and psychiatric symptoms were frequently endorsed and, while some symptoms such as hallucinations were unusual, several symptoms, such as laughing for no reason, inappropriate speech, and inappropriate affect, are consistent with ID and/or ASD features. ADHD symptoms, including attentional and impulse control challenges, were also often endorsed but not clearly elevated relative to the MA of participants.
Table 3 lists standardized behavioral/psychiatric measures used in PMS clinical case studies evaluating behavioral and psychiatric symptoms. Published reports used screening measures such as the Psychopathology Inventory for Mentally Retarded Adults (PIMRA; Matson, Kazdin, & Senatore, 1984), a clinician rated measure designed for use in adults with mild to moderate ID and the Developmental Behavior Checklist (DBC; Einfeld & Tonge, 1995), a caregiver report form designed for children and adults up to age 22. Shaw et al (2011) utilized additional measures including the Children’s Interview for Psychiatric Symptoms-Parent Version (P-ChIPS) and the Reiss Scales for Dual Diagnosis (Fristad, Teare, Weller, Weller, & Salmon, 1998; Reiss & Valenti-Hein, 1990). While these behavioral and psychiatric measures provide useful information, most measures rely on caregiver reports, and none of the reported measures have been normed in individuals with severe to profound ID.
Table 3.
Behavioral and psychiatric assessments reported in PMS studies
| Measure | Standardized Age Range | Administration Method | Description and Domains | PMS Studies: Cohort Descriptions & Clinically Relevant Findings |
|---|---|---|---|---|
| Psychopathological Instrument for Mentally Retarded Adults (PIMRA) (Matson, Kazdin, & Senatore, 1984) | Adult; psychometrics tested in ID sample | Parent/caregiver interview by clinician; 15 minutes |
DSM-III diagnostic categories: Schizophrenia, Affective disorder, Psychosexual, disorder, Adjustment disorder, Anxiety disorder, Somatoform disorder, Personality disorder, Inappropriate adjustment | No normed scores for diagnostic purposes; (n=1) (Verhoeven et al., 2013) |
| No normed scores for diagnostic purposes; (n=2) (Verhoeven et al., 2012) | ||||
| Aberrant Behavior Checklist (ABC) (Aman, Singh, Stewart, & Field, 1985) | Children and adults; psychometrics tested in ID sample | Parent/Caregiver rated; 20 minutes |
Irritability, Lethargy, Hyperactivity, Inappropriate speech, Stereotypy | Mean social withdrawal score: 16.9/48; (n=9) (Kolevzon, Bush, et al., 2014) |
| Developmental, Behavior Checklist (DBC) (Einfeld & Tonge, 1995) | 4–18 yrs.; psychometrics tested in ID sample | Parent/caregiver or teacher rated; 20 minutes | Self-absorbed, Disruptive, Communication disturbance, Social relating, Anxiety | Scores N/A (n=7) (Denayer et al., 2012) |
| Parent Rated Strengths and Difficulties Questionnaire (PSDQ) (Goodman, 1997) | 2–17 yrs. | Parent/caregiver rated; 5–10 minutes |
Screening for: Emotional symptoms, Conduct problems, Hyperactivity/inattention, Peer relationships, Prosocial behaviors | Subjects above the ADHD cutoff score: 9 (n=25) (Jeffries et al., 2005) |
| Children’s Interview for Psychiatric Syndromes-Parent Version (P-ChIPS) (Fristad et al., 1998) | 6–18 yrs. | Parent interview by clinician 20–50 minutes |
Attention-deficit/hyperactivity disorder, Oppositional, defiant disorder, Conduct disorder, Substance abuse, Specific phobia, Social phobia, Separation anxiety disorder, Generalized anxiety disorder, Obsessive-compulsive disorder, Stress disorders, Anorexia/-Bulimia, Depression/dysthymia, Mania/hypnosis, Enuresis/Encopresis, Schizophrenia/psychosis, Psychosocial stressors | Scores N/A (n=35) (Shaw, 2011) |
| The Reiss Scales for Children’s Dual Diagnosis (Reiss & Valenti-Hein, 1990) | 4–21 years | Parent/caregiver rated; 10 minutes |
Anger/self-control problems, Anxiety disorder, Attention-deficit disorder, Autism/pervasive developmental disorder, Conduct disorder, Depression, Poor self-esteem, Psychosis, Somatoform behavior, Withdrawn-isolated behavior | Subscales for which the mean was above the cutoff score: Psychosis, Depression, Attention Deficit; (n=35) (Shaw, 2011) |
| Repetitive Behavior Scale – Revised (RBS-R) (Bodfish et al., 2000) | 6–17 years; (psychometrics tested in ID sample) | Parent/caregiver rated; 15 minutes |
Stereotyped, Self-injurious, Compulsive, Routine, Sameness, Restricted Behavior | Mean Total Score: 32.1/129; (n=9) (Kolevzon, Bush, et al., 2014) |
| Bush Francis Catatonia Rating Scale (BFCRS)(Bush, Fink, Petrides, Dowling, & Francis, 1996) | Children, adolescents and adults | Clinician administered; 30–45 minutes | 23 symptoms associated with catatonia | Scores N/A; (n=2) (Serret et al., 2015) |
AE = age equivalent
ADHD = attention deficit/hyperactivity disorder
DSM-III = Diagnostic and Statistical Manual of Mental Disorders, Third Edition
ID = Intellectual Disability
Mos. = months
N/A = not available or not reported
PDD = pervasive developmental disorder
PMS = Phelan-McDermid Syndrome
Yrs. = years
Selecting behavioral & psychiatric measures
Clinical assessment of behavioral and psychiatric comorbidities is a critical element of the neuropsychological evaluation process in rare genetic disorders and PIMD. Rates of psychiatric comorbidities within intellectual disability range from 10–71% (Belva & Matson, 2014), with increased severity of ID and age associated with higher rates of psychiatric comorbidities across neurodevelopmental disorders (Belva & Matson, 2014). Common psychiatric conditions in ID include ADHD, anxiety disorders, and mood disorders, in addition to ASD, although very few studies have examined these rates exclusively in individuals with severe to profound ID (Forster, Gray, Taffe, Einfeld, & Tonge, 2011).
As seen in other domains, standardized clinical assessment is limited by conceptual and psychometric challenges. In the PMS literature, behavioral and psychiatric measurement often includes tools designed for individuals with mild to moderate IDs; an approach that underestimates the influence of verbal status, sensory impairments, and developmental level on interpretation of behaviors topographically similar to psychotic, anxiety, and mood disorders. The Aberrant Behavior Checklist (Aman, Singh, Stewart, & Field, 1985) is one of the few measures normed in individuals with ID and assesses functionally impairing behaviors associated with irritability, hyperactivity, stereotyped behavior, social withdrawal, and aggression.
The use of behavior analytic tools is strongly recommended in the clinical care of individuals with rare genetic conditions such as PMS. FBAs are the principal assessment tools in behavior analysis and provide a structure for assessment, treatment-planning, and monitoring. Though costly in time, FBAs yield objective data that overcome conceptual limitation of psychiatric diagnoses to young or developmentally delayed individuals – and have a long history in PIMD populations. Information is collected on common antecedents and consequences associated with a behavior in FBAs. Results are used to identify situations and conditions that predict the occurrence and non-occurrence of problem behavior (e.g. when, where, why). Hypotheses on the potential functions for a problem behavior (e.g. to escape from difficult tasks, to gain social attention) are formed and used to develop a treatment plan which is then monitored using the same data-collection strategy developed during the evaluation/assessment stage. The utility of FBAs in treating problem behaviors has led to more efficient and effective treatment for children with disabilities (O’Neill, et al., 1997) and has been mandated by the Individuals with Disabilities Education Act (IDEA, 2004) for use in the treatment of problem behaviors in students with disabilities in educational settings.
Discussion
This review highlights a dearth of validated measures as well as a reliance on modified testing procedures for the neurocognitive and behavioral assessment of individuals with PIMD. While the field of neuropsychological assessment adapts to provide reliable, objective, standardized measures for individuals with PIMD, practitioners may use the interdisciplinary, multi-modal framework outlined above to facilitate decision-making. A combination of standardized, direct and informant-report instruments (often modified) with functional assessments and clinical observation are recommended to characterize individuals with PIMD, monitor progress and develop treatment plans.
This framework supports the use of standardized measures, despite limitations, in the evaluation of PIMD associated with rare genetic conditions. Standardized tools will help advance the research base on PIMD and develop alternative approaches that can be used to advance investigations of conditions such as PMS. When standardized measures are used, practitioners are advised to document and describe the ways in which they depart from standardization. The addition of unconventional methods to neuropsychological assessment mirrors current educational practices that shift from standardized to functional assessment strategies such as response to intervention (RTI: Fuchs & Fuchs, 2006) for progress monitoring. RTI is a multi-tier approach to education that combines screening of an individual relative to same-aged peers, a stepped care model of intervention for specific skills (e.g. reading, math), and frequent curriculum based measurement (CBM) procedures to facilitate responsiveness to changes (positive, stagnant, or adverse). In PIMD, the use of systematic and skill-based assessment methods has similar advantages to RTI. At present, few tools are available to support curriculum-based assessments in PIMD. The Assessment of Basic Language and Learning Skills-Revised (ABLLS-R; Partington, 2008) is a widely used measure developed for children with ASD covering approximately two dozen cognitive, language, and adaptive skills domains relevant to daily functioning. Use in PIMD and PMS is limited but should be considered in both clinical care and progress monitoring in PIMD associated with rare genetic conditions. The combination of standardized and functional approaches inform personalized treatment and monitoring plans with capacity to identify incremental changes, and developmental lags, as well as regressions (Browder & Cooper-Duffy, 2003; O’Neill et al., 1997).
Research directions in neuropsychological evaluation of PIMD associated with genetic conditions
The development of psychometrically sound measures for PIMD is a critical step to supporting the rapidly evolving science of translational interventions for rare neurodevelopmental diseases. In PMS, clinical trials have already begun (Kolevzon, Bush, et al., 2014) and will need to use mental age-adjusted and mental age-appropriate metrics of development across critical neuropsychological assessment domains that may be treatment targets. In addition, measures will need to provide finer grained analyses of behavior to accommodate slower developmental trajectories in PIMD, and importantly, be useful for tracking change in target domains in translational research (Berry-Kravis et al., 2013). We now present a summary of domain-specific tools and conceptual approaches for further advancement of such neuropsychological evaluation approaches underway.
Motor and Sensory
Drawing from literature on conditions more purely defined by motor impairments, there is a precedent for more systematic and quantitative assessment of motor delay, impairment and dysfunction that may be applied to PIMD. For instance, the Gross Motor Function Classification System (GMFCS) (Rosenbaum, Palisano, Bartlett, Galuppi, & Russell, 2008), and instruments such as the Gross Motor Function Measure (Russell, Rosenbaum, Avery, & Lane, 2002), utilized in cerebral palsy may be tested and found applicable to PIMD. One tool specifically designed for systematic evaluation of motor impairments within this population is now under development, the Motor evaluation in Kids with Intellectual and Complex disabilities (Movakic; Mensch, Echteld, Evenhuis, & Rameckers, 2016), which measures 21 motor items over 12 situations. In addition, questionnaires using a functional approach to quantifying mobility such as the Pediatric Evaluation of Disability, Computerized Adaptive Test PEDI-CAT, may be applicable (Kao, Kramer, Liljenquist, Tian, & Coster, 2012).
Intellectual Functioning
Alternative methods of assessing concept formation and other basic cognitive tasks are now being developed, including computerized and tablet-based technology that provide promising opportunities to advance assessment strategies for individuals with PIMD (Chard, Roulin, & Bouvard, 2014). One example is an adaption of the NIH Toolbox, a computerized battery of cognitive and emotional domains, among others (Gershon et al., 2010; Weintraub, Bauer, et al., 2013), that was recently adapted to be usable in children with ID with MAs of 4 years and above (Hessl et al., 2016). Other paradigms have recently been developed, including a visual analog reasoning paradigm similar to a matrix reasoning test (Curie et al., 2016), as well as a test developed for individuals with significant motor impairment, expressive language limitations and cognitive MA less than 24 months (Leevers, Roesler, Flax, & Benasich, 2005).
Although not applicable for clinical use yet, researchers are developing novel strategies for intellectual assessment in the PIMD populations (Tenorio et al., 2014). For example studies are now starting to examine the use of gaze fixation to test underlying infant learning abilities in individuals with PIMD including processes such as visual habituation (Chard et al., 2014). Others have focused on methods for increasing motivation for responses such as gaming formats using touchscreen-based tasks (Delgado, Uribe, Alonso, & Diaz, 2016). Use of evoked potential EEG technology has also been considered to gauge cortical response, even when explicit responses (e.g. through eye fixation or manually indicated preference) are not achieved. For instance, studies of Rett syndrome have begun employing visual evoked potentials to directly assess cortical processing (LeBlanc et al., 2015). In cerebral palsy, auditory EEG paradigms have been used in conjunction with behavioral assessment, with some evidence for change directly related to treatment (Maitre et al., 2014).
Expressive and Receptive Language and Communication
Several lines of research have explored alternative methods to assess basic communication skills when motor impairments, cognitive level, or minimally verbal status limit traditional means for assessment (Cirrin & Rowland, 1985). Thus far, alternative language evaluations have primarily been evaluated in research settings (Tager-Flusberg et al., 2016), but have potential to be employed as augmentative or alternative communication strategies for clinical assessment (Brady et al., 2014). Some studies have evaluated eye tracking (Brady et al., 2014) to assess vocabulary on standardized measures such as the PPVT as well as other language skills (Chita-Tegmark, Arunachalam, Nelson, & Tager-Flusberg, 2015), with recent results indicating significant heterogeneity and thus, concerns about reliability across methods and measures (Plesa Skwerer, Jordan, Brukilacchio, & Tager-Flusberg, 2016).
Early communication skills in minimally verbal individuals are also important assessment domains with several experimental and emerging clinical tools reported in recent studies. For example, a pre-speech, experimental task has been used to determine preferences and sensitivities to speech versus non-speech sounds (Wang et al., 2016). The Communication Complexity Scale (CCS) is a clinical assessment designed exclusively for minimally verbal individuals throughout the lifespan (CCS; Brady et al., 2012). The CCS measures both early symbolic (e.g. beginning gestures, eye contact, and communicative behaviors integrated with eye contact), and pre-symbolic communicative behaviors and is being used in PIMD research including PMS. Alternatively, researchers have also utilized retrospective videotapes to code early communication development with the Inventory of Potential Communicative Acts (IPCA), which has been recently used in a study of Rett Disorder (Marschik et al., 2014).
Behavioral & Psychiatric Symptom Measures
Several psychiatric and behavioral symptoms measures are under development with potential clinical and research applications in rare genetic disorders associated with PIMD such as PMS. These recent reports are an encouraging indication of increased attention to the mental health needs of individuals with PIMD. Measures that have included severe or profound ID in the development process include: 1) The Problem Behavior Checklist (Tyrer et al., 2016), a 28 item Likert-type questionnaire developed to assess various aspects of challenging behavior identified by personal violence, violence against property, self-harm, sexually inappropriate, contrary, demanding and disappearing behavior, 2) the Challenging Behavior Interview (Oliver et al., 2003) a 2-part interview that was developed to assess the occurrence and severity of 5 different types of challenging behavior (i.e. self-injury, physical aggression, verbal aggression, disruption of the environment and inappropriate vocalization in both children and adults with severe ID, and 3) Behavior Problems Inventory (Rojahn, Matson, Lott, Esbensen, & Smalls, 2001), a 52-item rating scale developed for the intellectual disability population that measures the frequency and severity of 3 different problem behavior domains (i.e. self-injury, stereotyped behavior and aggressive/destructive behavior). In addition, there are several other measures available for the wider ID population (Deb & Unwin, 2007; National Collaborating Centre for Mental Health, 2015) although further testing is needed in all of these in exclusive samples of individuals with severe to profound ID in order to understand their utility in PIMD.
Summary
Advances in neuroscience and genetic discovery have led to the identification of rare genetic diseases associated with PIMD. Neurodevelopmental and neuropsychological assessment protocols tailored to this population are needed to inform clinical practice and guide scientific discovery. The field currently relies on instruments developed for - and standardized in - younger individuals and participants with higher intellectual functioning. When conducted, clinical assessment utilizes developmental scores, relies on caregiver reports and employs individualized assessment strategies (e.g. functional behavioral analysis).
New and refined instruments, sensitive to the subtle developmental trajectories of PIMD, are clearly needed to guide treatment planning and measure change in treatment research. Technological advances such as computer-based, game-like paradigms and assistive technology are under development. However, piloting, standardizing, and preparing novel strategies for evaluating cognition and communication with strategies such as eye tracking to become “off-the-shelf” for clinics requires considerable time. This framework seeks to provide guidance while emerging strategies are developed and assist meaningful, comprehensive assessment and treatment plans to guide the care of individuals with PIMD.
Acknowledgments
We would like to thank Cristan Farmer for her contribution to this manuscript. This work was supported by the Intramural Research Program (1ZIAMH002868) of the National Institute of Mental Health through NCT01778504, protocol 13-M-0028. We would also like to thank the Developmental Synaptopathies Consortium (U54NS092090), part of NCATS Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Disease Research (ORDR). This consortium is funded through collaboration between NCATS, and the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Several terms have been used to describe this population. In addition to PMID, PMLD has also been used, and refers to Profound and Multiple Learning Disabilities.
References
- Aman MG, Singh NN, Stewart AW, & Field CJ (1985). The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency, 89(5), 485–491. [PubMed] [Google Scholar]
- American Psychiatric Association. (1980). DSM-III: Diagnostic and Statistical Manual of Psychiatric Disorders. APA, Washington. [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: Author. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Bayley N, & Reuner G (2006). Bayley scales of infant and toddler development: Bayley-III (Vol. 7): Harcourt Assessment, Psych. Corporation San Antonio, Tex, USA. [Google Scholar]
- Belva BC, & Matson JL (2014). Examining the psychometrics of the Psychopathology Inventory for Mentally Retarded Adults-II for individuals with mild and moderate intellectual disabilities. Res Dev Disabil, 36c, 291–302. doi: 10.1016/j.ridd.2014.10.017 [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Abbeduto L, Reiss AL, Beckel-Mitchener A, & Urv TK (2013). Outcome measures for clinical trials in fragile X syndrome. J Dev Behav Pediatr, 34(7), 508–522. doi: 10.1097/DBP.0b013e31829d1f20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelli MO, Salvador-Carulla L, Scuticchio D, Varrucciu N, Martinez-Leal R, Cooper S-A, … Walsh C (2014). Moving beyond intelligence in the revision of ICD-10: specific cognitive functions in intellectual developmental disorders. World Psychiatry, 13(1), 93–94. doi: 10.1002/wps.20094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur C, & Buxbaum JD (2013). SHANK3 haploinsufficiency: a “common” but underdiagnosed highly penetrant monogenic cause of autism spectrum disorders. Mol Autism, 4(1), 17. doi: 10.1186/2040-2392-4-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Farmer C, & Thurm A (2014). Measurement of Nonverbal IQ in Autism Spectrum Disorder: Scores in Young Adulthood Compared to Early Childhood. J Autism Dev Disord. doi: 10.1007/s10803-014-2250-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, & Lewis MH (2000). Varieties of Repetitive Behavior in Autism: Comparisons to Mental Retardation. J Autism Dev Disord, 30(3), 237–243. doi: 10.1023/a:1005596502855 [DOI] [PubMed] [Google Scholar]
- Bosch DG, Boonstra FN, Reijnders MR, Pfundt R, Cremers FP, & de Vries BB (2014). Chromosomal aberrations in cerebral visual impairment. Eur J Paediatr Neurol, 18(6), 677–684. doi: 10.1016/j.ejpn.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Brady NC, Anderson CJ, Hahn LJ, Obermeier SM, & Kapa LL (2014). Eye tracking as a measure of receptive vocabulary in children with autism spectrum disorders. Augment Altern Commun, 30(2), 147–159. doi: 10.3109/07434618.2014.904923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady NC, Fleming K, Thiemann-Bourque K, Olswang L, Dowden P, Saunders MD, & Marquis J (2012). Development of the communication complexity scale. Am J Speech Lang Pathol, 21(1), 16–28. doi: 10.1044/1058-0360(2011/10-0099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browder DM, & Cooper-Duffy K (2003). Evidence-based practices for students with severe disabilities and the requirement for accountability in “No Child Left Behind”. The Journal of Special Education, 37(3), 157–163. [Google Scholar]
- Bruininks R, Woodcock RW, Weatherman R, & Hill B (1996). Scales of Independent Behaviour–Revised. Boston, MA: Houghton Mifflin Harcourt. [Google Scholar]
- Chard M, Roulin JL, & Bouvard M (2014). Visual habituation paradigm with adults with profound intellectual and multiple disabilities: a new way for cognitive assessment? J Appl Res Intellect Disabil, 27(5), 481–488. [DOI] [PubMed] [Google Scholar]
- Chita-Tegmark M, Arunachalam S, Nelson CA, & Tager-Flusberg H (2015). Eye-tracking measurements of language processing: developmental differences in children at high risk for ASD. J Autism Dev Disord, 45(10), 3327–3338. doi: 10.1007/s10803-015-2495-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrin FM, & Rowland CM (1985). Communicative assessment of nonverbal youths with severe/profound mental retardation. Ment Retard, 23(2), 52–62. [PubMed] [Google Scholar]
- Curie A, Brun A, Cheylus A, Reboul A, Nazir T, Bussy G, … des Portes V (2016). A Novel Analog Reasoning Paradigm: New Insights in Intellectually Disabled Patients. PLoS One, 11(2), e0149717. doi: 10.1371/journal.pone.0149717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S, & Unwin G (2007). Guide to using psychotropic medication to manage behaviour problems among adults with intellectual disability: Technical document: Birmingham: University of Birmingham, Mencap and the Royal College of Psychiatrists. [Google Scholar]
- Delgado MT, Uribe PA, Alonso AA, & Diaz RR (2016). TENI: A comprehensive battery for cognitive assessment based on games and technology. Child Neuropsychol, 22(3), 276–291. doi: 10.1080/09297049.2014.977241 [DOI] [PubMed] [Google Scholar]
- Denayer A, Van Esch H, de Ravel T, Frijns JP, Van Buggenhout G, Vogels A, … Swillen A (2012). Neuropsychopathology in 7 Patients with the 22q13 Deletion Syndrome: Presence of Bipolar Disorder and Progressive Loss of Skills. Molecular Syndromology, 3(1), 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar SU, del Gaudio D, German JR, Peters SU, Ou Z, Bader PI, … Sahoo T (2010). 22q13.3 Deletion Syndrome: Clinical and Molecular Analysis Using Array CGH. American Journal of Medical Genetics. Part A, 152A(3), 573–581. doi: 10.1002/ajmg.a.33253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2013). Executive Functions. Annual Review of Psychology, 64(1), 135–168. doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, & Dunn DM (2007). Peabody Picture Vocabulary Test, 4th edition. [Google Scholar]
- Egger JI, Zwanenburg RJ, van Ravenswaaij-Arts CM, Kleefstra T, & Verhoeven WM (2016). Neuropsychological phenotype and psychopathology in seven adult patients with Phelan-McDermid syndrome: implications for treatment strategy. Genes Brain Behav, 15(4), 395–404. doi: 10.1111/gbb.12285 [DOI] [PubMed] [Google Scholar]
- Einfeld SL, & Tonge BJ (1995). The Developmental Behavior Checklist: The development and validation of an instrument to assess behavioral and emotional disturbance in children and adolescents with mental retardation. Journal of Autism and Developmental Disorders, 25(2), 81–104. doi: 10.1007/bf02178498 [DOI] [PubMed] [Google Scholar]
- Elliott CD (Ed.). (2007). Manual for the Differential Ability Scales, Second Edition San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Fenson L, Marchman VA, Thal DJ, Dale PS, Reznick JS, & E., B. (2007). The MacArthur-Bates Communicative Development Inventories User’s Guide and Technical Manual, Second Edition. Baltimore, MD: Brookes. [Google Scholar]
- Forster S, Gray KM, Taffe J, Einfeld SL, & Tonge BJ (2011). Behavioural and emotional problems in people with severe and profound intellectual disability. Journal of Intellectual Disability Research, 55(2), 190–198. doi: 10.1111/j.1365-2788.2010.01373.x [DOI] [PubMed] [Google Scholar]
- Fristad MA, Teare M, Weller EB, Weller RA, & Salmon P (1998). Study III: development and concurrent validity of the Children’s Interview for Psychiatric Syndromes--parent version (P-ChIPS). J Child Adolesc Psychopharmacol, 8(4), 221–226. doi: 10.1089/cap.1998.8.221 [DOI] [PubMed] [Google Scholar]
- Fuchs D, & Fuchs LS (2006). Introduction to response to intervention: What, why, and how valid is it? Reading Research Quarterly, 41(1), 93–99. [Google Scholar]
- Gadalla KK, Ross PD, Riddell JS, Bailey ME, & Cobb SR (2014). Gait analysis in a mecp2 knockout mouse model of rett syndrome reveals early-onset and progressive motor deficits. PLoS ONE, 9(11), e112889. doi: 10.1371/journal.pone.0112889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RC, Cella D, Fox NA, Havlik RJ, Hendrie HC, & Wagster MV (2010). Assessment of neurological and behavioural function: the NIH Toolbox. The Lancet Neurology, 9(2), 138–139. [DOI] [PubMed] [Google Scholar]
- Gong X, Jiang YW, Zhang X, An Y, Zhang J, Wu Y, … Wang H (2012). High proportion of 22q13 deletions and SHANK3 mutations in Chinese patients with intellectual disability. PLoS One, 7(4), e34739. doi: 10.1371/journal.pone.0034739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R (1997). The Strengths and Difficulties Questionnaire: a research note. Journal of Child Psychology and Psychiatry, 38(5), 581–586. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, … Reynolds A (2008). Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology, 22(1), 48–60. doi: 10.1037/0894-4105.22.1.48 [DOI] [PubMed] [Google Scholar]
- Han Q, Kim YH, Wang X, Liu D, Zhang ZJ, Bey AL, … Ji RR (2016). SHANK3 Deficiency Impairs Heat Hyperalgesia and TRPV1 Signaling in Primary Sensory Neurons. Neuron, 92(6), 1279–1293. doi: 10.1016/j.neuron.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JC (2016). The origin and natural history of autism spectrum disorders. Nat Neurosci, 19(11), 1390–1391. doi: 10.1038/nn.4427 [DOI] [PubMed] [Google Scholar]
- Harrison P, & Oakland T (2015). Adaptive Behavior Assessment System, Third Edition (ABAS-3). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Havdahl KA, Hus Bal V, Huerta M, Pickles A, Øyen A-S, Stoltenberg C, … Bishop SL (2016). Multidimensional Influences on Autism Symptom Measures: Implications for Use in Etiological Research. Journal of the American Academy of Child & Adolescent Psychiatry, 55(12), 1054–1063.e1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Sansone SM, Berry-Kravis E, Riley K, Widaman KF, Abbeduto L, … Gershon RC (2016). The NIH Toolbox Cognitive Battery for intellectual disabilities: three preliminary studies and future directions. J Neurodev Disord, 8(1), 35. doi: 10.1186/s11689-016-9167-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes-Bernstein J, & Waber DP (1990). Developmental neuropsychological assessment: The systemic approach. Neuropsychology, 311–371. [Google Scholar]
- Hus V, Maye M, Harvey L, Guthrie W, Liang J, & Lord C (2011). The adapted ADOS—Preliminary findings using a modified version of the ADOS for adults who are nonverbal or have limited language. Paper presented at the Poster presented at the International Meeting for Autism Research, San Diego, CA. [Google Scholar]
- Jeffries AR, Curran S, Elmslie F, Sharma A, Wenger S, Hummel M, & Powell J (2005). Molecular and phenotypic characterization of ring chromosome 22. Am J Med Genet A, 137(2), 139–147. doi: 10.1002/ajmg.a.30780 [DOI] [PubMed] [Google Scholar]
- Kao YC, Kramer JM, Liljenquist K, Tian F, & Coster WJ (2012). Comparing the functional performance of children and youths with autism, developmental disabilities, and no disability using the revised pediatric evaluation of disability inventory item banks. Am J Occup Ther, 66(5), 607–616. doi: 10.5014/ajot.2012.004218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasari C, Brady N, Lord C, & Tager-Flusberg H (2013). Assessing the Minimally Verbal School-Aged Child With Autism Spectrum Disorder. Autism Research, 6(6), 479–493. doi: 10.1002/aur.1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolevzon A, Angarita B, Bush L, Wang AT, Frank Y, Yang A, … Buxbaum JD (2014). Phelan-McDermid syndrome: a review of the literature and practice parameters for medical assessment and monitoring. J Neurodev Disord, 6(1), 39. doi: 10.1186/1866-1955-6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolevzon A, Bush L, Wang AT, Halpern D, Frank Y, Grodberg D, … Buxbaum JD (2014). A pilot controlled trial of insulin-like growth factor-1 in children with Phelan-McDermid syndrome. Mol Autism, 5(1), 54. doi: 10.1186/2040-2392-5-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouser M, Speed HE, Dewey CM, Reimers JM, Widman AJ, Gupta N, … Xiao B (2013). Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission. The Journal of Neuroscience, 33(47), 18448–18468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraijer D, & de Bildt A (2005). The PDD-MRS: an instrument for identification of autism spectrum disorders in persons with mental retardation. Journal of Autism and Developmental Disorders, 35(4), 499–513. doi: 10.1007/s10803-005-5040-0 [DOI] [PubMed] [Google Scholar]
- LeBlanc JJ, DeGregorio G, Centofante E, Vogel-Farley VK, Barnes K, Kaufmann WE, … Nelson CA (2015). Visual evoked potentials detect cortical processing deficits in Rett syndrome. Ann Neurol, 78(5), 775–786. doi: 10.1002/ana.24513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CS, Nava C, Polge A, Gauthier J, Huguet G, Lumbroso S, … Bourgeron T (2014). Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: a gradient of severity in cognitive impairments. PLoS Genet, 10(9), e1004580. doi: 10.1371/journal.pgen.1004580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers HJ, Roesler CP, Flax J, & Benasich AA (2005). The Carter Neurocognitive Assessment for children with severely compromised expressive language and motor skills. J Child Psychol Psychiatry, 46(3), 287–303. doi: 10.1111/j.1469-7610.2004.00354.x [DOI] [PubMed] [Google Scholar]
- Liang SG, Sadovnick AD, Remick RA, Keck PE, McElroy SL, & Kelsoe JR (2002). A linkage disequilibrium study of bipolar disorder and microsatellite markers on 22q13. Psychiatric Genetics, 12(4), 231–235. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism Diagnostic Observation Schedule–2nd edition (ADOS-2). Los Angeles, CA: Western Psychological Corporation. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Mahone EM, & Slomine BS (2008). Neurodevelopmental disorders Textbook of clinical neuropsychology, 105–127. [Google Scholar]
- Maitre NL, Henderson G, Gogliotti S, Pearson J, Simmons A, Wang L, … Key AP (2014). Feasibility of event-related potential methodology to evaluate changes in cortical processing after rehabilitation in children with cerebral palsy: a pilot study. J Clin Exp Neuropsychol, 36(7), 669–679. doi: 10.1080/13803395.2014.925094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschik PB, Bartl-Pokorny KD, Tager-Flusberg H, Kaufmann WE, Pokorny F, Grossmann T, … Einspieler C (2014). Three different profiles: early socio-communicative capacities in typical Rett syndrome, the preserved speech variant and normal development. Dev Neurorehabil, 17(1), 34–38. doi: 10.3109/17518423.2013.837537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson JL, Kazdin AE, & Senatore V (1984). Psychometric properties of the psychopathology instrument for mentally retarded adults. Applied Research in Mental Retardation, 5(1), 81–89. doi: 10.1016/S0270-3092(84)80021-1 [DOI] [PubMed] [Google Scholar]
- Maulik PK, Mascarenhas MN, Mathers CD, Dua T, & Saxena S (2011). Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil, 32(2), 419–436. doi: 10.1016/j.ridd.2010.12.018 [DOI] [PubMed] [Google Scholar]
- Mensch SM, Echteld MA, Evenhuis HM, & Rameckers EA (2016). Construct validity and responsiveness of Movakic: An instrument for the evaluation of motor abilities in children with severe multiple disabilities. Res Dev Disabil, 59, 194–201. doi: 10.1016/j.ridd.2016.08.012 [DOI] [PubMed] [Google Scholar]
- Messias E, Kaley SN, & McKelvey KD (2013). Adult-Onset Psychosis and Clinical Genetics: A Case of Phelan-McDermid Syndrome. The Journal of Neuropsychiatry and Clinical Neurosciences, 25(4), E27–E27. doi: 10.1176/appi.neuropsych.12100241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieses AM, Tavassoli T, Li E, Soorya L, Lurie S, Wang AT, … Kolevzon A (2016). Brief Report: Sensory Reactivity in Children with Phelan–McDermid Syndrome. J Autism Dev Disord, 46(7), 2508–2513. doi: 10.1007/s10803-016-2754-0 [DOI] [PubMed] [Google Scholar]
- Moss J, & Howlin P (2009). Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res, 53(10), 852–873. doi: 10.1111/j.1365-2788.2009.01197.x [DOI] [PubMed] [Google Scholar]
- Mullen EM (Ed.). (1995). Mullen Scales of Early Learning. Circle Pines, MN American Guidance Service. [Google Scholar]
- Munde V, & Vlaskamp C (2015). Initiation of activities and alertness in individuals with profound intellectual and multiple disabilities. J Intellect Disabil Res, 59(3), 284–292. doi: 10.1111/jir.12138 [DOI] [PubMed] [Google Scholar]
- Nakken H, & Vlaskamp C (2007). A Need for a Taxonomy for Profound Intellectual and Multiple Disabilities. Journal of Policy and Practice in Intellectual Disabilities, 4(2), 83–87. doi: 10.1111/j.1741-1130.2007.00104.x [DOI] [Google Scholar]
- National Collaborating Centre for Mental Health. (2015). National Institute for Health and Care Excellence: Clinical Guidelines Challenging Behaviour and Learning Disabilities: Prevention and Interventions for People with Learning Disabilities Whose Behaviour Challenges. London: National Institute for Health and Care Excellence (UK) [PubMed] [Google Scholar]
- Newborg J, Stock JR, Wnek L. 1984. Battelle Developmental Inventory. Allen, TX: DLM Teaching Resources. [Google Scholar]
- O’Neill R, Horner R, Albin R, Sprague J, Storey K, & Newton J (1997). Functional assessment and program development for problem behavior. Pacific Grove, CA: Brooks: Cole Publishing. [Google Scholar]
- Oberman LM, Boccuto L, Cascio L, Sarasua S, & Kaufmann WE (2015). Autism spectrum disorder in Phelan-McDermid syndrome: initial characterization and genotype-phenotype correlations. Orphanet J Rare Dis, 10, 105. doi: 10.1186/s13023-015-0323-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterling J, & Dawson G (1994). Early recognition of children with autism: A study of first birthday home videotapes. J Autism Dev Disord, 24(3), 247–257. doi: 10.1007/bf02172225 [DOI] [PubMed] [Google Scholar]
- Partington JW (2008). The assessment of basic language and learning skills-revised (the ABLLS-R). Walnut Hill, CA: Behavior Analysts. [Google Scholar]
- Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, … Feng G (2011). Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature, 472(7344), 437–442. doi: 10.1038/nature09965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan MC, Rogers RC, Saul RA, Stapleton GA, Sweet K, McDermid H, … Kelly DP (2001). 22q13 deletion syndrome. Am J Med Genet, 101(2), 91–99. [DOI] [PubMed] [Google Scholar]
- Philippe A, Boddaert N, Vaivre-Douret L, Robel L, Danon-Boileau L, Malan V, … Munnich A (2008). Neurobehavioral profile and brain imaging study of the 22q13.3 deletion syndrome in childhood. Pediatrics, 122(2), e376–382. doi: 10.1542/peds.2007-2584 [DOI] [PubMed] [Google Scholar]
- Philippe A, Craus Y, Rio M, Bahi-Buisson N, Boddaert N, Malan V, … Robel L (2015). Case report: an unexpected link between partial deletion of the SHANK3 gene and Heller’s dementia infantilis, a rare subtype of autism spectrum disorder. BMC Psychiatry, 15, 256. doi: 10.1186/s12888-015-0631-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesa Skwerer D, Jordan SE, Brukilacchio BH, & Tager-Flusberg H (2016). Comparing methods for assessing receptive language skills in minimally verbal children and adolescents with autism spectrum disorders. Autism, 20(5), 591–604. doi: 10.1177/1362361315600146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppes P, van der Putten AJJ, Post WJ, & Vlaskamp C (2016). Risk factors associated with challenging behaviour in people with profound intellectual and multiple disabilities. Journal of Intellectual Disability Research, 60(6), 537–552. doi: 10.1111/jir.12268 [DOI] [PubMed] [Google Scholar]
- Redin C, Gérard B, Lauer J, Herenger Y, Muller J, Quartier A, … Piton A (2014). Efficient strategy for the molecular diagnosis of intellectual disability using targeted high-throughput sequencing. Journal of Medical Genetics, 51(11), 724–736. doi: 10.1136/jmedgenet-2014-102554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S, & Valenti-Hein D (1990). The Reiss scales for children’s dual diagnosis. Chicago: IDS. [Google Scholar]
- Reynell J, & Gruber G (1984). Reynell Developmental Language Scales (RDLS)-Second version: British edition London: NFER-Nelson. [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, … Pickles A (2006). Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of American Academy of Child & Adolescent Psychiatry, 45(9), 1094–1103. [DOI] [PubMed] [Google Scholar]
- Roid GH, & Miller LJ (2011). Leiter International Performance Scale-Revised (Leiter-R). Madrid: Psymtec. [Google Scholar]
- Rosenbaum PL, Palisano RJ, Bartlett DJ, Galuppi BE, & Russell DJ (2008). Development of the gross motor function classification system for cerebral palsy. Developmental Medicine & Child Neurology, 50(4), 249–253. [DOI] [PubMed] [Google Scholar]
- Russell DJ, Rosenbaum PL, Avery LM, & Lane M (2002). Gross motor function measure (GMFM-66 and GMFM-88) user’s manual: Cambridge University Press. [Google Scholar]
- Rutter M, LeCouteur A, & Lord C (2003). Autism Diagnostic Interview-Revised (ADI-R). Los Angeles, California: Western Psychological Services. [Google Scholar]
- Sappok T, Diefenbacher A, Budczies J, Schade C, Grubich C, Bergmann T, … Dziobek I (2013). Diagnosing autism in a clinical sample of adults with intellectual disabilities: how useful are the ADOS and the ADI-R? Res Dev Disabil, 34(5), 1642–1655. doi: 10.1016/j.ridd.2013.01.028 [DOI] [PubMed] [Google Scholar]
- Sarasua SM, Boccuto L, Sharp JL, Dwivedi A, Chen CF, Rollins JD, … DuPont BR (2014). Clinical and genomic evaluation of 201 patients with Phelan-McDermid syndrome. Hum Genet, 133(7), 847–859. doi: 10.1007/s00439-014-1423-7 [DOI] [PubMed] [Google Scholar]
- Sarasua SM, Dwivedi A, Boccuto L, Rollins JD, Chen CF, Rogers RC, … Collins JS (2011). Association between deletion size and important phenotypes expands the genomic region of interest in Phelan-McDermid syndrome (22q13 deletion syndrome). J Med Genet, 48(11), 761–766. doi: 10.1136/jmedgenet-2011-100225 [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler R, Bashford A, Lansing M, & Marcus L (1990). Individual Assessment and Treatment for Autistic and Developmental Disabled Children Vol 1 Psychoeducational profile revised (PEP-R) Austin: Pro-Ed. Austin, TX: Pro-Ed. [Google Scholar]
- Serret S, Thummler S, Dor E, Vesperini S, Santos A, & Askenazy F (2015). Lithium as a rescue therapy for regression and catatonia features in two SHANK3 patients with autism spectrum disorder: case reports. BMC Psychiatry, 15, 107. doi: 10.1186/s12888-015-0490-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SRR, Amira; Sharma, Akanksha. (2011). Behavioral Profiles in Phelan-McDermid Syndrome: Focus on Mental Health. Journal of Mental Health Research in Intellectual Disabilities, 4, 1–18. [Google Scholar]
- Simon EW, Haas-Givler B, & Finucane B (2013). An Introduction to Genetic Intellectual Disability Syndromes. Genetic Syndromes and Applied Behaviour Analysis: A Handbook for ABA Practitioners, 17. [Google Scholar]
- Siper PM, Kolevzon A, Wang AT, Buxbaum JD, & Tavassoli T (2017). A clinician-administered observation and corresponding caregiver interview capturing DSM-5 sensory reactivity symptoms in children with ASD. Autism Res, 10(6), 1133–1140. doi: 10.1002/aur.1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders JT, Tellegen PJ, & Laros JA (1989). Snijders-Oomen Non-verbal intelligence test: SON-R 5, 5–17. Manual and research reportWolters-Noordhoff Groningen. [Google Scholar]
- Soorya L, Kolevzon A, Zweifach J, Lim T, Dobry Y, Schwartz L, … Buxbaum JD (2013). Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol Autism, 4(1), 18. doi: 10.1186/2040-2392-4-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S,S, Cicchetti DV, & Saulnier C (2016). Vineland Adaptive Behavior Scales, Third Edition (Vineland-3). San Antonio, TX: Pearson Assessment. [Google Scholar]
- Sparrow SS, Carter AS, & Cicchetti DV (1993). Vineland Screener: Overview, reliability, validity, administration, and scoring. New Haven, CT: Yale University Child Study Center. [Google Scholar]
- Sparrow SS, Cicchetti DV, & Balla DA (2005). Vineland Adaptive Behavior Scales, Second Edition. Circle Pines, MN: AGS Publishing. [Google Scholar]
- Tager-Flusberg H, Plesa Skwerer D, Joseph RM, Brukilacchio B, Decker J, Eggleston B, … Yoder A (2016). Conducting research with minimally verbal participants with autism spectrum disorder. Autism. doi: 10.1177/1362361316654605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Rogers S, Cooper J, Landa R, Lord C, Paul R, … Yoder P (2009). Defining Spoken Language Benchmarks and Selecting Measures of Expressive Language Development for Young Children with Autism Spectrum Disorders. J Speech Lang Hear Res. doi: 1092-4388_2009_08-0136 [pii] 10.1044/1092–4388(2009/08–0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenorio M, Campos R, & Karmiloff-Smith A (2014). What standardized tests ignore when assessing individuals with neurodevelopmental disorders/Lo que ignoran los tests estandarizados en la evaluación de personas con trastornos del neurodesarrollo. Estudios de psicologia, 35(2), 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm A, Tierney E, Farmer C, Albert P, Joseph L, Swedo S, … Porter FD (2016). Development, behavior, and biomarker characterization of Smith-Lemli-Opitz syndrome: an update. J Neurodev Disord, 8, 12. doi: 10.1186/s11689-016-9145-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino S, & Waga C (2013). SHANK3 as an autism spectrum disorder-associated gene. Brain and Development, 35(2), 106–110. [DOI] [PubMed] [Google Scholar]
- Van der Molen MJ, Huizinga M, Huizenga HM, Ridderinkhof KR, Van der Molen MW, Hamel BJ, … Ramakers GJ (2010). Profiling Fragile X Syndrome in males: strengths and weaknesses in cognitive abilities. Res Dev Disabil, 31(2), 426–439. doi: 10.1016/j.ridd.2009.10.013 [DOI] [PubMed] [Google Scholar]
- Verhoeven WM, Egger JI, Cohen-Snuijf R, Kant SG, & de Leeuw N (2013). Phelan–McDermid syndrome: Clinical report of a 70-year-old woman. American Journal of Medical Genetics Part A, 161(1), 158–161. [DOI] [PubMed] [Google Scholar]
- Verhoeven WM, Egger JI, Willemsen MH, de Leijer GJ, & Kleefstra T (2012). Phelan-McDermid syndrome in two adult brothers: atypical bipolar disorder as its psychopathological phenotype? Neuropsychiatr Dis Treat, 8, 175–179. doi: 10.2147/ndt.s30506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Kubendran S, Das SK, Jain S, & Brahmachari SK (2005). SYNGR1 is associated with schizophrenia and bipolar disorder in southern India. Journal of Human Genetics, 50(12), 635–640. doi: 10.1007/s10038-005-0307-z [DOI] [PubMed] [Google Scholar]
- Vucurovic K, Landais E, Delahaigue C, Eutrope J, Schneider A, Leroy C, … Doco-Fenzy M (2012). Bipolar affective disorder and early dementia onset in a male patient with SHANK3 deletion. Eur J Med Genet, 55(11), 625–629. doi: 10.1016/j.ejmg.2012.07.009 [DOI] [PubMed] [Google Scholar]
- Wang AT, Lim T, Jamison J, Bush L, Soorya LV, Tavassoli T, … Kolevzon A (2016). Neural selectivity for communicative auditory signals in Phelan-McDermid syndrome. J Neurodev Disord, 8, 5. doi: 10.1186/s11689-016-9138-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warschausky S, Van Tubbergen M, Asbell S, Kaufman J, Ayyangar R, & Donders J (2012). Modified test administration using assistive technology: preliminary psychometric findings. Assessment, 19(4), 472–479. doi: 10.1177/1073191111402458 [DOI] [PubMed] [Google Scholar]
- Weintraub S, Bauer PJ, Zelazo PD, Wallner-Allen K, Dikmen SS, Heaton RK, … Gershon RC (2013). I. NIH Toolbox Cognition Battery (CB): introduction and pediatric data. Monogr Soc Res Child Dev, 78(4), 1–15. doi: 10.1111/mono.12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, … Gershon RC (2013). Cognition assessment using the NIH Toolbox. Neurology, 80(11 Suppl 3), S54–64. doi: 10.1212/WNL.0b013e3182872ded [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker S (2008). The merits of mental age as an additional measure of intellectual ability in the low ability range. Paper presented at the Clinical Psychology Forum. [Google Scholar]
- Wright CF, Fitzgerald TW, Jones WD, Clayton S, McRae JF, van Kogelenberg M, … Firth HV (2015). Genetic diagnosis of developmental disorders in the DDD study: a scalable analysis of genome-wide research data. Lancet, 385(9975), 1305–1314. doi: 10.1016/s0140-6736(14)61705-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink I, & Lembrechts D (2000). Nederlandstalige Nonspeech Test (vertaling en hernormering van de Nonspeech Test van Mary Blake Huer). Leuven: Acco. [Google Scholar]
- Zwanenburg RJ, Ruiter SAJ, van den Heuvel ER, Flapper BCT, & Van Ravenswaaij-Arts CMA (2016). Developmental phenotype in Phelan-McDermid (22q13.3 deletion) syndrome: a systematic and prospective study in 34 children. J Neurodev Disord, 8(1), 16. doi: 10.1186/s11689-016-9150-0 [DOI] [PMC free article] [PubMed] [Google Scholar]