Extended Data Fig. 10 |. Model of TRAIP’s action in interphase and mitosis.
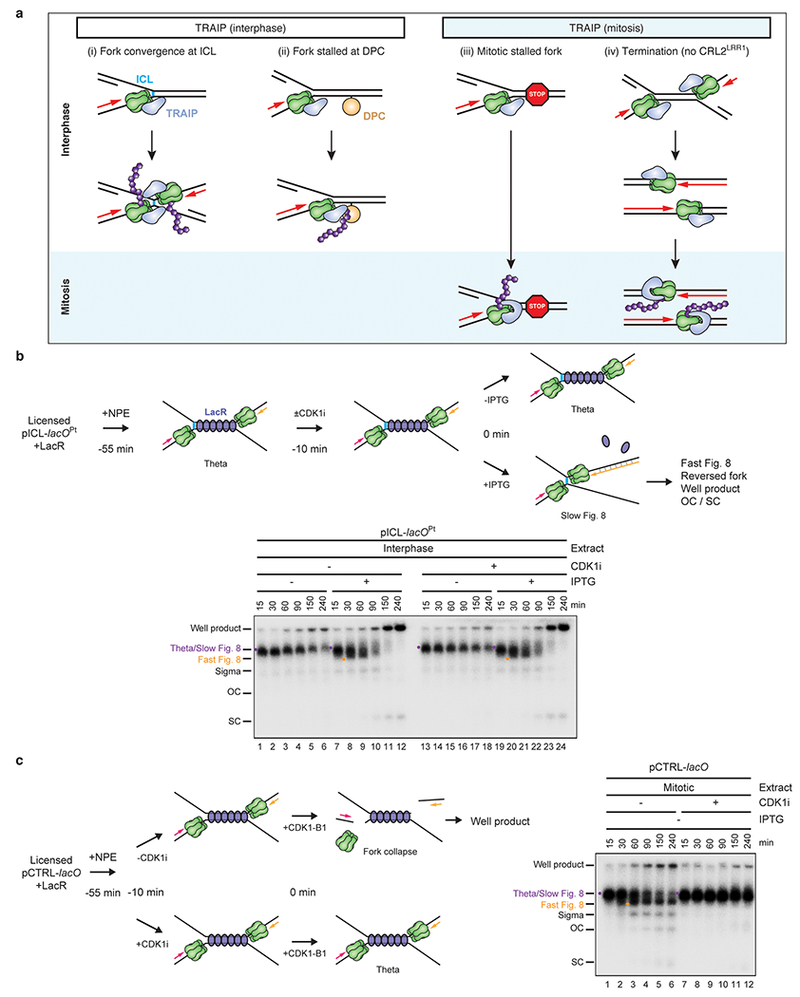
a, TRAIP (blue) travels with the replisome and associates with CMG, directly or indirectly (i-iv, top). In interphase, TRAIP is oriented such that its E2 (whose identity is unknown) points ahead of the replisome. As a result, TRAIP cannot ubiquitylate the CMG it is associated with, but it can ubiquitylate any protein that lies in the replisome’s path. TRAIP can thus ubiquitylate CMG in trans when two replisomes meet (i), and it can ubiquitylate DPCs that block the path of the replisome (ii). We speculate that TRAIP ubiquitylates any proteinaceous structure that causes extended stalling of the replisome. In mitosis, TRAIP undergoes a conformational change so that it can ubiquitylate the CMG with which it is associated in cis. As a result, in mitosis, stalled forks undergo TRAIP-dependent CMG ubiquitylation in the absence of fork convergence (iii) (Deng et al., in press). We propose that TRAIP does not ubiquitylate terminated CMGs in interphase because they rapidly move past each other29, precluding ubiquitylation by the forward-pointing TRAIP (iv, middle). However, upon mitotic entry, terminated CMGs undergo TRAIP-dependent ubiquitylation in cis (iv, bottom)(Deng et al., in press). Importantly, TRAIP-dependent CMG unloading in interphase egg extracts is not dependent on residual CDK1 activity (b and c), indicating that TRAIP is regulated differently in interphase and mitosis.
b, In interphase egg extracts, TRAIP travels with DNA replication forks but ubiquitylates CMGs only when forks converge. In the presence of Cyclin B1-CDK1, TRAIP is activated in the absence of fork convergence (Deng et al., in press)(summarized in a). We therefore wanted to know whether TRAIP-dependent CMG unloading in interphase egg extract depends on residual CDK1 activity. Top, reaction scheme to determine whether the action of TRAIP at ICLs in interphase egg extract requires residual CDK1 activity. Replication of pICL-lacOPt with a pre-assembled LacR array was initiated by addition of NPE (−55 min). Forty-five min after initiation (−10 min), reactions were supplemented with buffer or the CDK1 inhibitor RO-3306 (CDK1i) and allowed to incubate for an additional 10 min; CDK1i was added late to avoid inhibition of replication initiation. The LacR array was then released with addition of IPTG to trigger fork convergence and ICL repair (0 min). In a control, buffer instead of IPTG was added to maintain the LacR array and thereby prevent fork convergence. At the indicated times after IPTG addition, samples were collected and analyzed as in Fig. 1b to look for evidence of CMG unloading and ICL processing. Bottom, experiment described in top scheme. Fork stalling at the boundaries of the LacR array lead to a theta structure (lanes 1, 7, 13, 19). Upon addition of IPTG, theta was converted to Slow Figure 8, Fast Figure 8 (co-migrating with theta), and well product with equal efficiency in the presence and absence of CDK1i (compare lanes 7-12 and 19-24). The data suggest that CDK1 is not required for CMG unloading or ICL repair.
c, Left, reaction scheme of a control experiment to ensure that the addition of CDK1i in b blocked CDK1 activity. As recently described (Deng et al., in press), when replication forks are stalled at a LacR array, the subsequent addition of Cyclin B1-CDK1 promotes TRAIP-dependent CMG unloading and fork breakage, alternative end joining, and the formation of aberrant replication products that migrate in the well of an agarose gel. To verify that CDK1 was effectively inhibited in a, we used this CDK1-dependent well-product formation as an assay. To this end, replication of pCTRL-lacO with a pre-assembled LacR array was initiated (−55 min) by addition of NPE. Forty-five min after initiation (−10 min), reactions were supplemented with CDK1i or buffer and allowed to incubate for an additional 10 min. Cyclin B1-CDK1 was then added to activate fork breakage and well product formation (0 min). Right, experiment described in left scheme. As expected, addition of Cyclin B1-CDK1 in the absence of CDK1i led to the formation of well products (lanes 25-30) but not in the presence of CDK1i (lanes 31-36). Note that the experiments in b and c were performed in parallel; extracts were aliquoted for the various conditions described at 0 min. Therefore, the CDK1i effectively inhibited CDK1-dependent activation of TRAIP. We conclude that TRAIP activation at ICLs does not depend on residual CDK1 activity.
