Extended Data Fig. 3 |. TRAIP, but not FANCM, ATR, or BRCA1, is required for CMG unloading at cisplatin-ICLs.
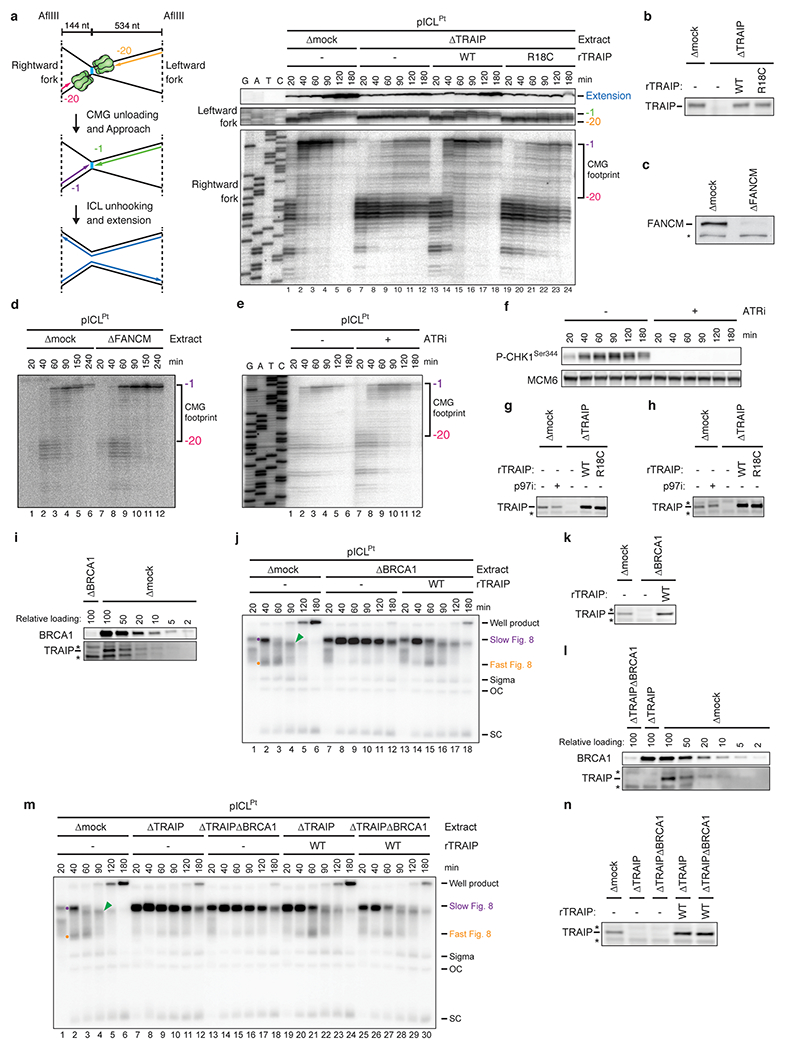
a, Left, schematic of nascent strands generated at ICLs. When forks converge on an ICL, nascent strands stall ~20 nt from the ICL on either side of the lesion due to the footprint of CMG (green hexamer). AflIII cuts 144 nt to the left and 534 nt to the right of the ICL, generating characteristic products for the leftward and rightward leading strands upon fork convergence, CMG unloading, and leading strand extension. Right, nascent strand analysis of pICLPt replication in the indicated extracts. After replication with [α-32P]dATP, nascent strands were extracted, digested with AflIII, and resolved on a denaturing polyacrylamide gel alongside a sequencing ladder and visualized by autoradiography. As seen previously2,51, when replication forks converged on the ICL in mock-depleted egg extracts, leading strands initially stalled 20-40 nucleotides (nt) from the lesion (lane 1) and then advanced to the −1 position (lanes 2-6), which depends on CMG dissociation19,51. In contrast, in TRAIP-depleted egg extracts, the −20 footprint persisted for three hours (lanes 7-12). This effect was rescued with rTRAIPWT but not rTRAIPR18C (lanes 13-24).
b, Extracts used in the replication reaction shown in a were blotted for TRAIP.
c, Mock- or FANCM-depleted extracts were blotted for FANCM. A non-specifically detected protein is marked with an asterisk.
d, Nascent strand analysis of pICLPt replicating in mock- or FANCM-depleted extracts was performed as in a. The CMG footprint disappeared at the ICL in FANCM-depleted egg extract, consistent with FANCM not being required for CMG unloading at ICLs.
e, pICLPt was replicated in the absence or presence of ATR inhibitor ETP-46464 (ATRi), and nascent strand analysis was performed as in a. ATR inhibitor was added to the reaction 2.5 min after initiation. The CMG footprint disappeared at the ICL with or without ATR inhibitor, indicating that ATR signaling is not required for CMG unloading at ICLs.
f, Extracts used in e were sampled at various time points and blotted for Xenopus CHK1 Serine-344 phosphorylation to verify ATR inhibition. MCM6 was detected as a loading control.
g and h, Mock- and TRAIP-depleted extracts used in one of the replicate reactions quantified in Fig. 1e (g) and f (h) were blotted for TRAIP.
i, We previously showed that the immunodepletion of BRCA1 from egg extracts inhibits CMG unloading at ICLs, but this defect could not be rescued with recombinant BRCA1-BARD1 complex6,19. To test whether TRAIP is co-depleted with BRCA1, NPE was immunodepleted of BRCA1 with BRCA1 antiserum, loaded alongside a dilution series of mock-depleted NPE, and blotted for BRCA1 and TRAIP. A relative loading amount of 100 corresponds to 2 µl of NPE. Non-specifically detected proteins are marked with asterisks. This analysis revealed that immunodepletion of BRCA1 also removed TRAIP from NPE. Notably, we also observed TRAIP co-depletion with antibodies against other proteins (data not shown), suggesting it interacts non-specifically with different antibodies.
j, The extracts described in i were supplemented with pICLPt, [α-32P]dATP, and rTRAIP, as indicated, and analyzed as in Fig. 1b. rTRAIPWT suppressed the stabilization of the Slow Figure 8 species seen in BRCA1-depleted extract, consistent with the restoration of CMG unloading, and indicating that the unloading defect seen in BRCA1-depleted egg extracts is due primarily to the removal of TRAIP from the extract.
k, The extracts used in j were blotted for TRAIP.
l, To determine whether BRCA1 contributes to TRAIP-dependent CMG unloading, NPE was immunodepleted of TRAIP or TRAIP and BRCA1 using Protein A Sepharose-purified antibodies purified from antiserum. A dilution series of mock-depleted NPE was loaded alongside the depleted extracts, and extracts were blotted for BRCA1 and TRAIP. A relative loading amount of 100 corresponds to 2 µl of NPE.
m, The extracts described in l were supplemented with pICLPt, [α-32P]dATP, and rTRAIP, as indicated, and analyzed as in Fig. 1b. rTRAIPWT suppressed the accumulation of Slow Figure 8s to a similar extent in TRAIP-depleted egg extracts whether or not BRCA1 was co-depleted (lanes 19-30), indicating that BRCA1 is not needed to support TRAIP function. BRCA1 depletion reproducibly resulted in a decrease in well product formation, suggesting a role for BRCA1 in recombination after a double-strand break is formed by ICL unhooking incisions.
n, The extracts used in m were blotted for TRAIP.
