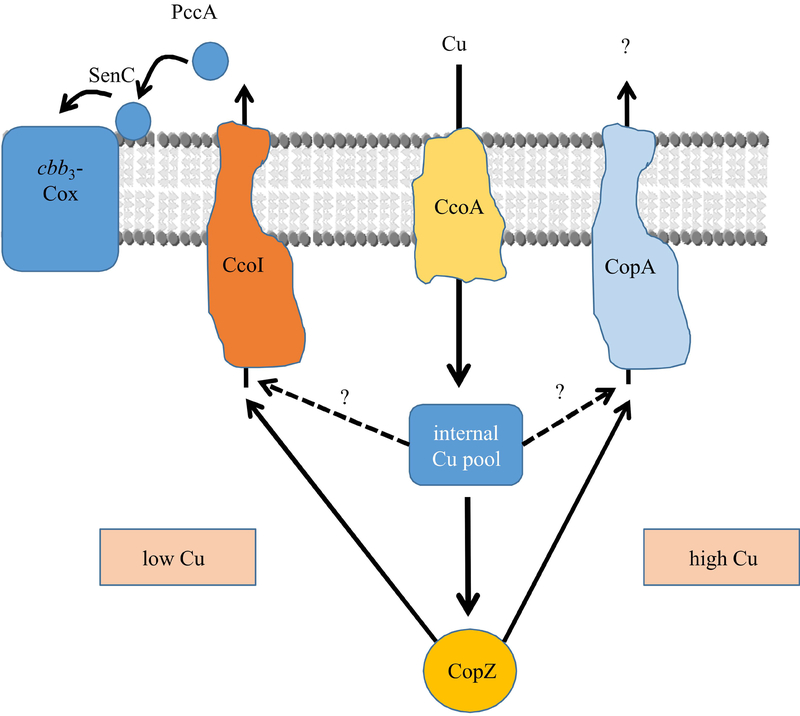
Fig. 8: The dual role of CopZ in R. capsulatus Cu homeostasis.
Cu uptake in R. capsulatus is primarily mediated by the major facilitator superfamily protein CcoA. Cytosolic Cu is present in a poorly defined internal Cu pool, and is rapidly transferred to CopZ. At low Cu concentrations, Cu supply to cbb3-Cox is maintained by both the internal Cu pool (dashed line) and by CopZ-bound Cu (solid line), both feeding Cu into the CcoI-dependent Cu export. Subsequently, Cu is bound by two periplasmic Cu chaperones, PccA and SenC, and transferred to cbb3-Cox. Thus, in the absence of CopZ, Cu supply to cbb3-Cox is reduced, but still possible via the internal Cu pool. Under these conditions, CopA does not significantly drain the internal Cu pool due to its lower Cu affinity.
At high Cu concentrations, Cu supply for cbb3-Cox assembly is also maintained by the internal Cu pool and by CopZ. But CopZ will predominantly feed Cu into the CopA-dependent export (solid line), and due to the combined activity of CopZ and CopA, the cellular Cu concentration will rapidly decrease and reach again low Cu concentrations. However, in the absence of CopZ, CopA can still receive some amounts of Cu from the increased internal Cu pool (dashed line), hence Cu detoxification is at least partially sustained even in the absence of CopZ. This working model rationalizes why the absence of CopZ confers less pronounced Cu sensitivity than the absence of CopA and why it decreases but not abolishes the cbb3-Cox activity.