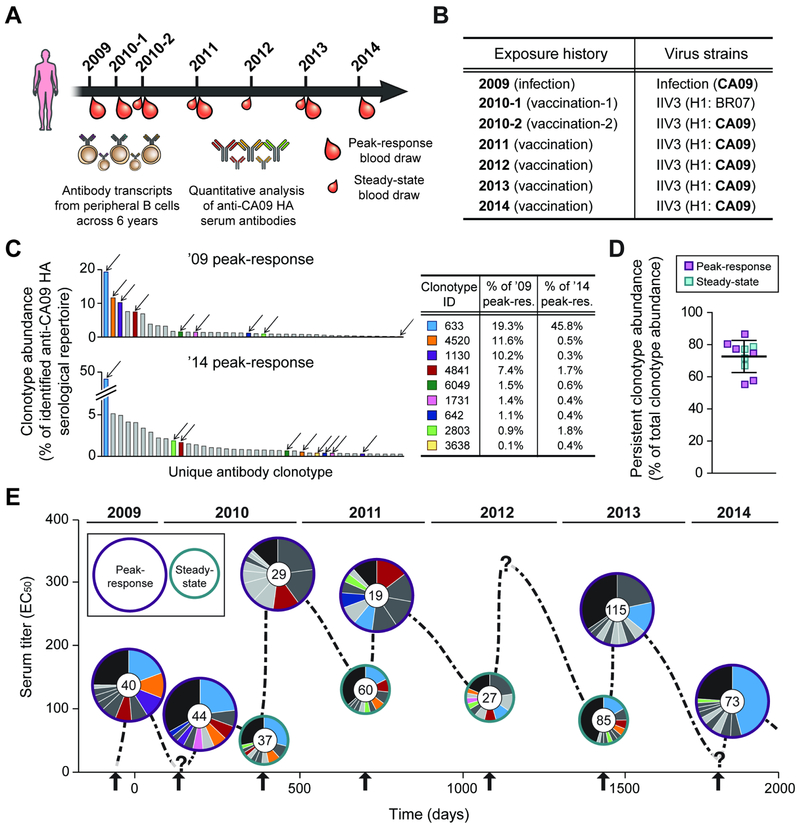
Fig. 1. Longitudinal analysis of CA09 HA-reactive serum antibodies from 2009 to 2014.
A. Serum samples were collected at the time points indicated and the serological repertoire at each time point was determined by Ig-Seq (see also Table 1). B. Exposure history of the donor to CA09. For each trivalent inactivated influenza vaccine (IIV3), H1 component is indicated. BR07, Brisbane/59/2007. C. Composition of the anti-CA09 HA serological repertoire at the first (’09 peak-response) and last (’14 peak-response) time points (see also Fig. S2 and Table S3). Each bar represents one of the 40 most abundant antibody clonotypes with height corresponding to the relative abundance of that clonotype. The 9 antibody clonotypes detected every year are indicated with arrows, and their relative abundances in the ’09 and ’14 peak-response sera are shown in the table. D. Relative abundance of the 24 persistent antibody clonotypes, detected in serum samples for ≥4 out of 5 years. Error-bars indicate s.d. (n = 10). E. The anti-CA09 HA antibody repertoire composition over 5 years (see also Table S2). y-axis shows the serum titer to CA09 HA. Within each pie chart, each of the top 10 most abundant antibody clonotypes is represented as a slice with an area proportional to the relative abundance of that clonotype. The 9 antibody clonotypes in C are shown using the same color scheme while the other 15 persistent antibody clonotypes are shown in darker grey. Anti-CA09 HA antibody clonotypes that are not among the top 10 most abundant antibodies are grouped together in the black slice. Inner circle in pie chart shows the number of antibody clonotypes identified in the serum sample. Arrows indicate exposure events. ‘?’, no serum sample was collected at that time point.