Abstract
Background
In Korea, the incidence of varicella has increased despite the introduction of a universal one-dose vaccination for children aged 12–15 months in 2005. A previous study demonstrated that the vaccine effectiveness was insufficient to prevent against varicella. We assessed the effect of the varicella vaccination on disease severity.
Methods
Epidemiologic investigation of varicella cases in Seoul metropolitan area from 2015 to 2017 were used. Varicella-related symptoms such as rash were determined by the clinical practitioners. Disease severity of patients was assessed by the number of skin lesions and divided into mild (≤ 50) and moderate (51–249) to severe (≥ 250). Unconditional logistic regression analysis was performed and age was controlled.
Results
Among a total of 1,008 varicella cases reported, 869 cases were breakthrough cases and 139 were unvaccinated cases. The risk for occurrence of moderate-to-severe disease in the breakthrough group was 0.57 times less than that of the unvaccinated group.
Conclusion
These data suggest that national varicella vaccination may have a significant effect on attenuation of disease severity in children.
Keywords: Varicella, Chickenpox, Vaccination, Breakthrough, Severity
Graphical Abstract
INTRODUCTION
Since the introduction of a universal varicella vaccination program, countries such as the United States, Germany, and Taiwan have experienced a reduced incidence rate of varicella.1,2,3 However, in Korea—where one-dose of varicella vaccination for all children aged 12–15 months was recommended by the National Immunization Program (NIP) in 2005, and the coverage had reached up to 98.9% in 20124—the incidence rate of varicella has been continuously rising from 22.5 per 100,000 persons to 154.8 from 2006 to 2017.5 A previous study demonstrated that the vaccine immunogenicity may be not sufficient to provide effective immunity against varicella infection.6,7
To our knowledge, there has been no population-based study to assess the disease severity of varicella cases after adoption of a universal one-dose varicella vaccination to the NIP. In this study, we aimed to investigate the effect of vaccination on the disease severity of varicella.
METHODS
Study population and data collection
In this study, 1,125 cases from 94 varicella outbreaks reported as part of epidemiologic investigation of varicella from January 2015 to December 2017 in the Seoul metropolitan area were used. Data were provided by the Korea Centers for Disease Control and Prevention (KCDC). According to the KCDC guidelines, an epidemiologic investigation is required if varicella outbreaks, including index cases, occur in more than 5% of students in a classroom within a 3-week period in schools, kindergartens, or day care centers. When a varicella outbreak is reported, public health centers should conduct an epidemiologic investigation within 3 days, collecting data from the patient's parent with the help of the school health teacher. The information includes the name, gender, date of birth, date of diagnosis, vaccination status, and related clinical symptoms such as rash (the number skin lesions), fever, headache, and arthralgia. Varicella-related symptoms were determined by the clinical practitioners through a physical examination of the patients. Disease severity of patients was assessed by the number of skin lesions as used in a previous study.8,9,10,11
Mild cases were defined as those having less than or equal to 50 skin lesions; moderate cases were defined as those having 51–249 skin lesions; and severe cases were defined as those having more than or equal to 250 skin lesions. We excluded 117 cases: 3 cases were excluded because the patient was infected before 12 months of age; 12 cases were excluded for having varicella within 42 days after the vaccination (breakthrough varicella is infection with varicella-zoster virus occurring in a vaccinated person more than 42 days after vaccination); 4 were excluded for being vaccinated with 2 doses of vaccine; and 98 cases because the patient was born before 2004.
Statistical analysis
We used t-test and χ2 to compare the difference in distribution in general characteristics, clinical symptoms, and disease severity in the vaccinated (breakthrough) and unvaccinated groups. Binary unconditional logistic regression analysis was performed to examine the differences in disease severity between the two groups, controlling for the factor of age. All statistical analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, NC, USA). P < 0.05 was considered significant, and all tests of statistical significance were two-sided.
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board (IRB) of Seoul National University (IRB No. E-1808-062-965). Informed consent was waived by the board because this was a retrospective study.
RESULTS
Among a total of 1,008 varicella cases in Seoul, Korea, 869 cases (86.2%) were breakthrough cases and 139 (13.8%) were unvaccinated cases. The mean age for the breakthrough cases was younger than for the unvaccinated cases (7.72 vs. 8.82 years; P < 0.001). No significant differences were observed in gender and reporting source distributions between the two groups. Of these cases, about half were male and 83% to 88% cases were elementary school students (Table 1).
Table 1. General characteristics of breakthrough cases and unvaccinated cases.
| Characteristics | Breakthrough cases (n = 869) | Unvaccinated cases (n = 139) | χ2 | P value | ||
|---|---|---|---|---|---|---|
| Age, yr | ||||||
| Mean ± SD | 7.72 ± 1.86 | 8.82 ± 2.04 | 6.33a | < 0.001 | ||
| Age group, No. (%) | 50.58 | < 0.001 | ||||
| 1–3 | 15 (1.7) | 2 (1.4) | ||||
| 4–6 | 210 (24.2) | 14 (10.1) | ||||
| 7–9 | 487 (56.0) | 62 (44.6) | ||||
| 10–12 | 157 (18.1) | 61 (43.9) | ||||
| Sex, No. (%) | 0.4972 | 0.481 | ||||
| Male | 478 (55.0) | 72 (51.8) | ||||
| Female | 391 (45.0) | 67 (48.2) | ||||
| Reporting source, No. (%) | 4.1068 | 0.128 | ||||
| Kindergarten | 131 (15.1) | 13 (9.4) | ||||
| Elementary school | 724 (83.3) | 122 (87.8) | ||||
| Unknown | 14 (1.6) | 4 (1.6) | ||||
SD = standard deviation.
at-value.
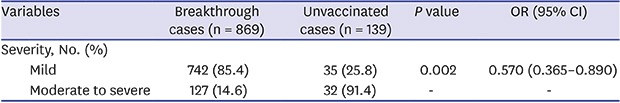
There was no difference in clinical symptoms between the two groups. Among patients, rash was the most common symptom in both the breakthrough and unvaccinated groups (99.5% and 100%) and rash onset was on most of the body (54.1% and 43.7%; P = 0.001). Fever was the second most common symptom (33.8% and 36.7%; P = 0.510); headache was the third most common (7.3% and 10.1%; P = 0.245); and arthralgia was a rare symptom (1.2%, only in the breakthrough group). Disease severity differed between the two groups. In the breakthrough group, the proportion of cases with moderate-to-severe symptoms was less than that in the unvaccinated group (14.6% vs. 25.8%; P = 0.002). The risk for occurrence of moderate-to-severe disease in the breakthrough group was less than roughly half that of the unvaccinated group (odds ratio [OR], 0.570; 95% confidence interval [CI], 0.365–0.890) (Table 2).
Table 2. Comparison of the epidemiology and disease severity between the breakthrough and unvaccinated cases.
| Variables | Breakthrough cases (n = 869) | Unvaccinated cases (n = 139) | P value | OR (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Clinical symptoms, No. (%)a | |||||||
| Rash | 865 (99.5) | 139 (100.0) | - | - | |||
| Affected part of rash onset | |||||||
| Face and neck | 333 (38.5) | 56 (40.3) | |||||
| Body | 468 (54.1) | 60 (43.2) | |||||
| Arms and legs | 64 (7.4) | 23 (16.5) | |||||
| Fever | 294 (33.8) | 51 (36.7) | 0.510 | 0.888 (0.602–1.309) | |||
| Headache | 63 (7.3) | 14 (10.1) | 0.245 | 0.975 (0.517–1.838) | |||
| Arthralgiab | 10 (1.2) | 0 (0.0) | - | - | |||
| Severity, No. (%) | |||||||
| Mild (< 50) | 742 (85.4) | 104 (74.8) | 0.002 | 0.570 (0.365–0.890) | |||
| Moderate to severe (≥ 50) | 127 (14.6) | 35 (25.8) | - | - | |||
| Moderate (50–249) | 114 (89.8) | 32 (91.4) | |||||
| Severe (≥ 250) | 13 (10.2) | 3 (8.6) | |||||
OR = odds ratio, CI = confidence interval.
aEvery clinical symptom was detected and recorded; bContinuity-adjusted χ2 test was used because one of the cell frequencies was equal to zero.
DISCUSSION
The results of the present study showed that a one-dose vaccination was associated with the attenuation of disease severity in pediatric varicella cases. We found that the risk of severe illness was significantly decreased in the breakthrough group compared with the unvaccinated group (14.6% vs. 25.8%; OR, 0.570). In other words, the vaccine effectiveness (1–OR) of one-dose of varicella vaccine administered at 12–15 months of age was 43% (95% CI, 11.0%–63.5%) against moderate-to-severe varicella.
This finding is consistent with results from previous studies reported elsewhere. An OR for illness that was of a severity more than mild in breakthrough cases than in unvaccinated cases was noted in Germany (12.5% vs. 68.2%; OR, 0.183),10 the United States (14.3% vs. 52.4%; OR, 0. 273),9 and China (25.6% vs. 44.8%; OR, 0.446).11
Our data may be contrasted with the results from studies on varicella vaccine effectiveness that suggest an insufficient immunogenicity of the vaccine in Korea. In a clinical-based study, the effectiveness of one varicella vaccine product (Suduvax, Green Cross, Seoul, Korea) was estimated as 54% (CI, 0.10–2.05) in a case-control study, and the seroconversion rate was 76.67% by the classical fluorescent antibody to membrane antigen assay.6 In a population-based study in Seoul, Korea, the effectiveness of the varicella vaccine was 13% (CI, −17.3–35.6) and the vaccine-induced immunity rapidly decreased three years after the vaccination, suggesting waning of the immunity.7
From these findings, we could suggest that a universal one-dose varicella vaccination program may have limited effectiveness to decrease in the incidence rate of varicella, but have positive effects in attenuating the disease severity in pediatric varicella cases. Two clinical studies on the severity of varicella in Korea demonstrated that a milder pattern of rash was observed in the breakthrough group versus the unvaccinated group12 and that the number of lesions detected were significantly fewer in the breakthrough group than in the unvaccinated group.13 From the perspective of a vaccinated patient, milder symptoms by attenuation of the disease severity are benefit. Contrarily, from the perspective of a population health care management, patients with breakthrough varicella can also transmit varicella to others despite that they generally have a lower rate of infectivity than those who are unvaccinated. Mild symptoms often lead to a failure to isolate patients and lead to outbreaks among those in close contact such as children in kindergarten or elementary school.
Our study has several limitations. A relatively small number of unvaccinated cases were identified due to a high level of vaccine coverage, which may cause selection bias. A tendency for a patient who is infected with varicella in their later years and develops severe disease could be confounded in our study because the mean age of the unvaccinated group is significantly higher than that of the breakthrough group (8.82 vs. 7.72 years). To alleviate this selection bias, however, we used age as a confounder in the logistic model. There could be also recall bias because the reporter would fill out the epidemiological survey depending on his or her memory. In addition, in the epidemiological survey form, the questionnaire about the number of lesions only requests the range of the number with a broad bracket (< 50, 50–249, 250–499, ≥ 500) rather than a concrete number of lesions, which makes it impossible to precisely distinguish disease severity. The exact impact of a universal one-dose varicella vaccination on disease severity could not be assessed due to a lack of previous population-based data on varicella vaccination and its effect on disease severity before adoption of the universal vaccination. Furthermore, we could not estimate changes in disease severity over time because there was no data on the annual incidence of varicella cases categorized by disease severity. Despite these limitations, the present study is the first population-based study to assess a universal one-dose varicella vaccination on disease severity in Korea after introduction of the national varicella vaccination program.
In conclusion, our study suggests that employing a universal one-dose varicella vaccination has had a significant effect on attenuating the disease severity in children. Additional research is necessary to assess the longitudinal effect of the varicella vaccination program on changing the epidemiology of varicella in Korea.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Lee YH, Choe YJ, Cho SI, Lee JK.
- Formal analysis: Lee YH.
- Methodology: Lee YH, Cho SI.
- Software: Lee YH.
- Supervision: Lee JK.
- Validation: Lee YH, Park H, Bang JH, Lee JK.
- Writing - original draft: Lee YH.
- Writing - review & editing: Lee YH, Choe YJ, Cho SI, Park H, Bang JH, Lee JK.
References
- 1.Seward JF, Watson BM, Peterson CL, Mascola L, Pelosi JW, Zhang JX, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995–2000. JAMA. 2002;287(5):606–611. doi: 10.1001/jama.287.5.606. [DOI] [PubMed] [Google Scholar]
- 2.Spackova M, Muehlen M, Siedler A. Complications of varicella after implementation of routine childhood varicella vaccination in Germany. Pediatr Infect Dis J. 2010;29(9):884–886. doi: 10.1097/INF.0b013e3181e2817f. [DOI] [PubMed] [Google Scholar]
- 3.Chang LY, Huang LM, Chang IS, Tsai FY. Epidemiological characteristics of varicella from 2000 to 2008 and the impact of nationwide immunization in Taiwan. BMC Infect Dis. 2011;11(1):352. doi: 10.1186/1471-2334-11-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choe YJ, Yang JJ, Park SK, Choi EH, Lee HJ. Comparative estimation of coverage between national immunization program vaccines and non-NIP vaccines in Korea. J Korean Med Sci. 2013;28(9):1283–1288. doi: 10.3346/jkms.2013.28.9.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korea Centers for Disease Control and Prevention. Korea Centers for Disease Control and Prevention National Notifiable Disease Surveillance System. [Updated 2018]. [Accessed August 10, 2018]. http://www.cdc.go.kr/npt.
- 6.Oh SH, Choi EH, Shin SH, Kim YK, Chang JK, Choi KM, et al. Varicella and varicella vaccination in South Korea. Clin Vaccine Immunol. 2014;21(5):762–768. doi: 10.1128/CVI.00645-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YH, Choe YJ, Cho SI, Kang CR, Bang JH, Oh MD, et al. Effectiveness of varicella vaccination program in preventing laboratory-confirmed cases in children in Seoul, Korea. J Korean Med Sci. 2016;31(12):1897–1901. doi: 10.3346/jkms.2016.31.12.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaves SS, Gargiullo P, Zhang JX, Civen R, Guris D, Mascola L, et al. Loss of vaccine-induced immunity to varicella over time. N Engl J Med. 2007;356(11):1121–1129. doi: 10.1056/NEJMoa064040. [DOI] [PubMed] [Google Scholar]
- 9.Vázquez M, LaRussa PS, Gershon AA, Steinberg SP, Freudigman K, Shapiro ED. The effectiveness of the varicella vaccine in clinical practice. N Engl J Med. 2001;344(13):955–960. doi: 10.1056/NEJM200103293441302. [DOI] [PubMed] [Google Scholar]
- 10.Liese JG, Cohen C, Rack A, Pirzer K, Eber S, Blum M, et al. The effectiveness of varicella vaccination in children in Germany: a case-control study. Pediatr Infect Dis J. 2013;32(9):998–1004. doi: 10.1097/INF.0b013e31829ae263. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Yu Y, Zhang J, Kwan EP, Huang S, Wang Z, et al. One-dose vaccination associated with attenuated disease severity of adolescent and adult varicella cases in Beijing's Fengtai District. Hum Vaccin Immunother. 2014;10(8):2417–2420. doi: 10.4161/hv.29140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DJ, Park HS, Lee SY, Park KS, Kim TK, Song YH, et al. Epidemiology of varicella in Korea based on pediatrician's office practice. J Korean Pediatr Soc. 1997;40(5):620–628. [Google Scholar]
- 13.Kim MR, Park JS, Kim DH, Lee HR, Park CY. A clinical and epidemiologic study on varicella in children. Pediatr Infect Vaccine. 1998;5(1):88–95. [Google Scholar]


