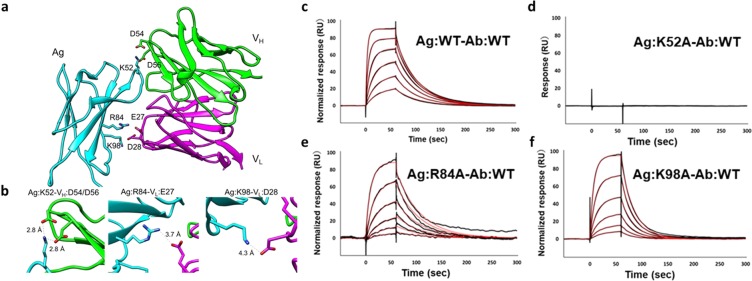
Figure 1.
Electrostatic interactions in the IFNγR-A6 complex and the SPR response profiles of the wild-type and the alanine mutant complexes. (a) Crystal structure of the antigen (cyan) and antibody (H chain: green, L chain: magenta) complex (from PDB ID: 1JRH, only variable regions are shown). (b) Electrostatic interactions at the interface of the wild-type complex. Each distance of electrostatic interaction is displayed as dash lines and was measured based on the crystal structure. Antibody variable regions are numbered based on the Kabat numbering scheme. SPR response profiles of (c) Ag:WT-Ab:WT, (d) Ag:K52A-Ab:WT (e) Ag:R84A-Ab:WT, and (f) Ag:K98A-Ab:WT. The concentrations of the antibody were 1000, 500, 250, 125, 62.5, and 31.25 nM. Raw data are in black; red lines are fitted curves. All fitted data were normalized and assume a 1:1 interaction. Binding kinetics were measured at 298 K.