Figure 1.
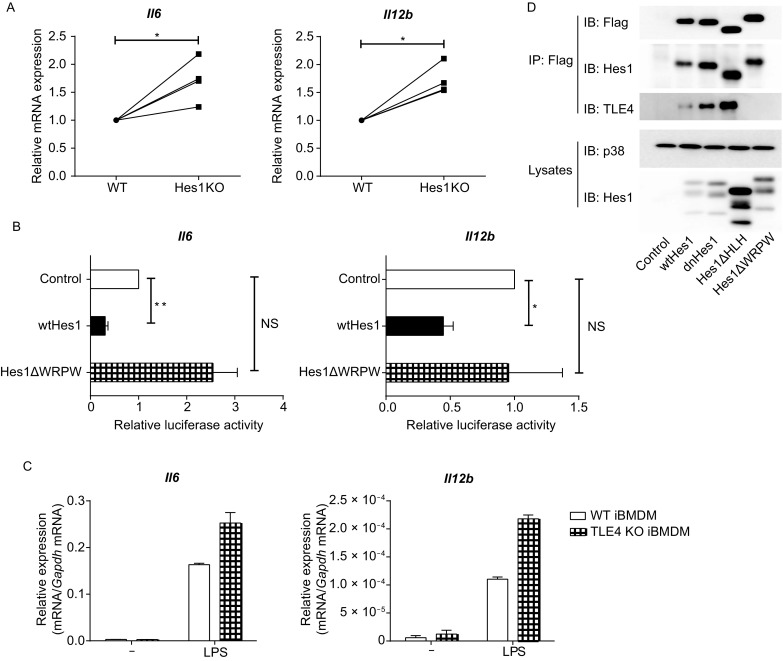
TLE4 is required for Hes1-mediated suppression of key pro-inflammatory gene expression. (A) Quantitative real-time PCR (qPCR) analysis of Il6 and Il12b mRNA in Mx1-Cre (WT) and Hes1fl/flMx1-Cre (Hes1 KO) BMDMs stimulated with 10 ng/mL of LPS for 3 h, presented relative to that in WT BMDMs. (B) Luciferase activities in RAW264.7 cells co-transfected with Il6 or Il12b promoter-driven reporter constructs and a Hes1 expression plasmid (pMx-Hes1, wtHes1), a Hes1 mutant expression plasmid (pMx-Hes1ΔWRPW, Hes1ΔWPRW) or control vector (pMx-GFP). 24 h post infection, cells were left untreated or stimulated with 100 ng/mL of LPS for 6 h, and cell lysates were analyzed for luciferase activities. Results are presented as ratio of luciferase activity/total protein concentration, and are normalized to values in the control vector group. (C) Quantitative real-time PCR analysis of Il6 and Il12b mRNA in wild type (WT) and TLE4 KO iBMDMs stimulated with or without LPS (100 ng/mL) for 3 h. (D) Immunoblot analysis of indicated proteins in immunoprecipitated (IP) samples and whole lysates of HEK 293T cells that overexpressed wild-type Hes1 or Hes1 mutants. p38 was used as a loading control. Data are representative of three (C) independent experiments (mean ± s.d. of technical triplicates), or are pooled from four (A) or three (B) independent experiments (mean ± s.d. of biological triplicates). ns, not significant. *P < 0.05, **P < 0.01 (Student’s paired t-test)
