Figure 6.
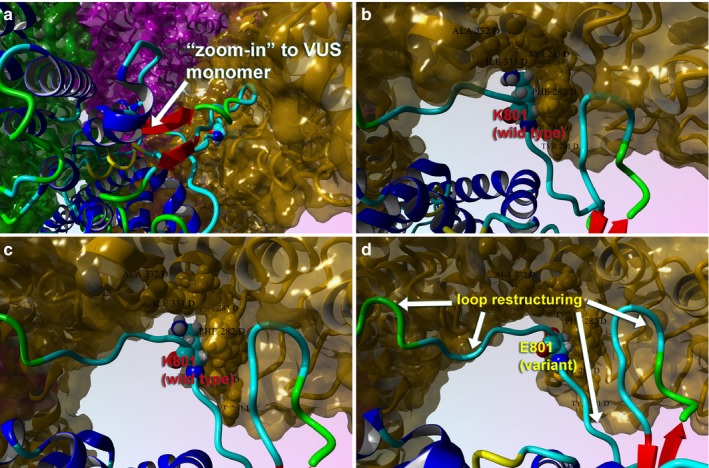
Close‐up views of the TRPV4 variant (p.K801E) surface and variant effect on loop structure and effect on tetrameric interface. (a) Full‐length model for the variant TRPV4 tetrameric structure colored by secondary structure and atom type. Interacting residues between the two proteins are shown. (b) The tetrameric structure for wild type (K801) is shown with same scheme. (c) Close‐up view of the loops is shown for the wild type in this monomer–monomer interaction region. (d) Same view for the p.K801E is shown to illustrate the changes in the loop conformation and the effect on tetramer conformation. This VUS causes the pore structure to be altered for the cation channel, which also demonstrates changes in the protein Gibbs‐free energy for stability
