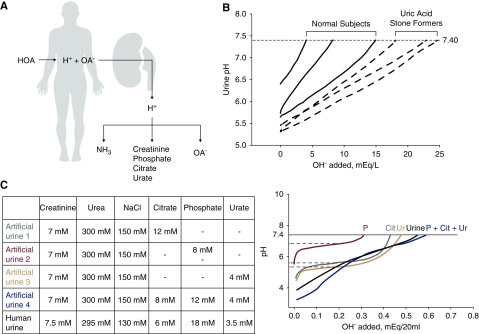
Figure 2.
Titration of human and artificial urine suggests that proton titration in the urine is accomplished by multiple buffers with wide overlapping ranges of acid dissociation constant (pKa) values. (A) Rationale for search for organic anions. High acid addition to the body results in high net acid excretion by the kidney. The H+ is carried by the conventional urinary buffers—ammonia (NH3), creatinine, phosphate, citrate, and urate. Inadequate ammoniagenesis/excretion leads to obligatory buffering by (B) 20 ml of spot urine from three normal subjects, and three subjects with idiopathic uric acid nephrolithiasis were titrated with dropwise addition of 10 μl 0.1 N NaOH until urine pH was 7.40. (C) Urinary titration was performed on artificial urine with the compositions shown in the table in the upper panel and compared with human urine. Titration curves are shown in the lower panel. Although ΔpH/NaOH added can identify values approximated by the acid dissociation constant (pKa) values of single buffers, such as phosphate, citrate, and urate, when all three buffers are added to artificial urine, the titration curve exhibits a smooth and almost constant slope, resembling real human urine. HOA, organic acid; OA-, organic anion.