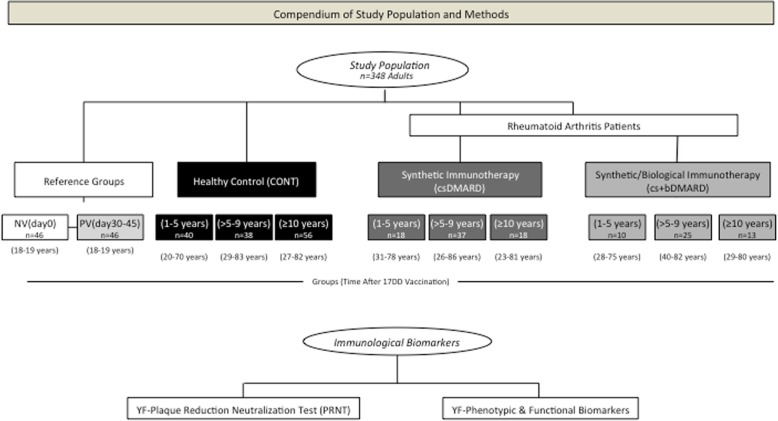
Fig. 1.
Compendium of the study population. A total of 348 adults were enrolled in the present investigation. One hundred and twenty-one adult RA patients with previous records of 17DD-YF vaccination were enrolled. Patients were first categorized into two subgroups, referred to as synthetic immunotherapy (csDMARD) or combined immunotherapy (cs+bDMARD) based on whether they were under current treatment with DMARDs or DMARDs combined with TNF-α inhibitors (adalimumab/ADA, certolizumab/CTZ, etanercept/ETN, golimumab/GOL, or infliximab/IFX), IL-6 antagonists (tocilizumab/TCZ), T lymphocyte co-stimulation modulators (abatacept/ABT), or anti-B-cell mAbs (rituximab/RTX); the patients were further categorized according to the time after 17DD vaccination as follows: csDMARD (1–5 years), csDMARD (> 5–9 years), csDMARD (≥ 10 years), and cs+bDMARD (1–5 years), cs+bDMARD (> 5–9 years), cs+bDMARD (≥ 10 years). The control group of the healthy subjects included 226 participants categorized into five subgroups referred as non-vaccinated subjects NV(day0), PV(day30–45), and three groups of volunteers, categorized according to the time after 17DD-YF vaccination and referred to as CONT(1–5 years), CONT(> 5–9 years), and CONT(≥ 10 years). Detailed descriptions of the study groups are provided in the “Methods” section. Immunological biomarker analyses, including YF plaque-reduction neutralization test (PRNT) and YF phenotypic and functional biomarkers, were performed for each participant