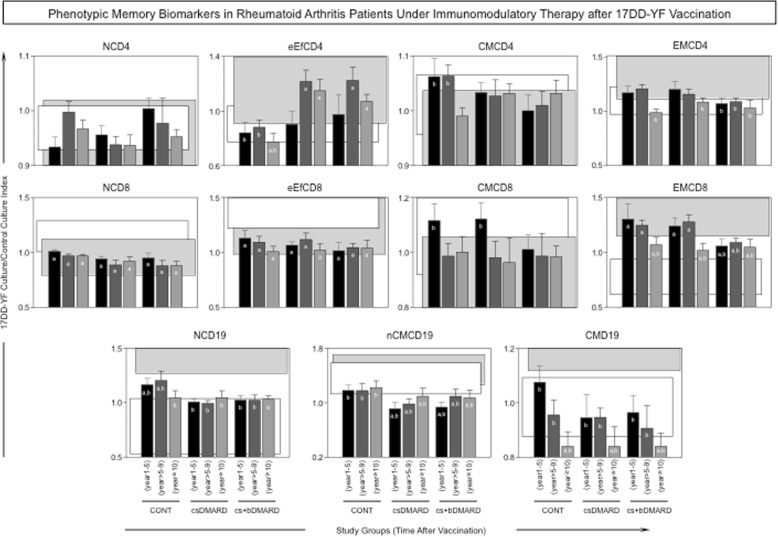
Fig. 3.
Phenotypic memory biomarkers in patients undergoing immunomodulatory therapy after 17DD-YF vaccination. An in vitro 17DD-YF-specific peripheral blood lymphoproliferative assay was employed for antigen recall. Flow cytometric assay were carried out for distinct memory T cell subsets, including naïve/(NCD4 and NCD8)/CD27+CD45RO−, early effector memory/(eEfCD4 and eEfCD8)/CD27−CD45RO−, central memory/(CMCD4 and CMCD8)/CD27+CD45RO+, and effector memory/(EMCD4 and EMCD8)/CD27−CD45RO+, along with memory B cell subsets, including naïve/(NCD19)/CD27−IgD+, non-classical memory/(nCMCD19)/CD27+IgD+, and classical memory/(CMCD19)/CD27+IgD−. The results are expressed as 17DD-YF Ag/CC index as described in the “Methods” section. Comparative analyses with the reference groups NV(day0) and PV(day30–45) were carried out using the mean value observed for each study group CONT(1–5 years), CONT(> 5–9 years), CONT(≥10 years), csDMARD(1–5 years), csDMARD(> 5–9 years), csDMARD(≥10 years), cs+bDMARD(1–5 years), cs+bDMARD(> 5–9 years), and cs+bDMARD(≥10 years) in comparison to the 95% CI of the reference groups, including NV(day0) (white rectangles) and PV(day30–45) (gray rectangles). Significant differences were highlighted by letters “a” and “b” compared to NV(day0) or PV(day30–45), respectively