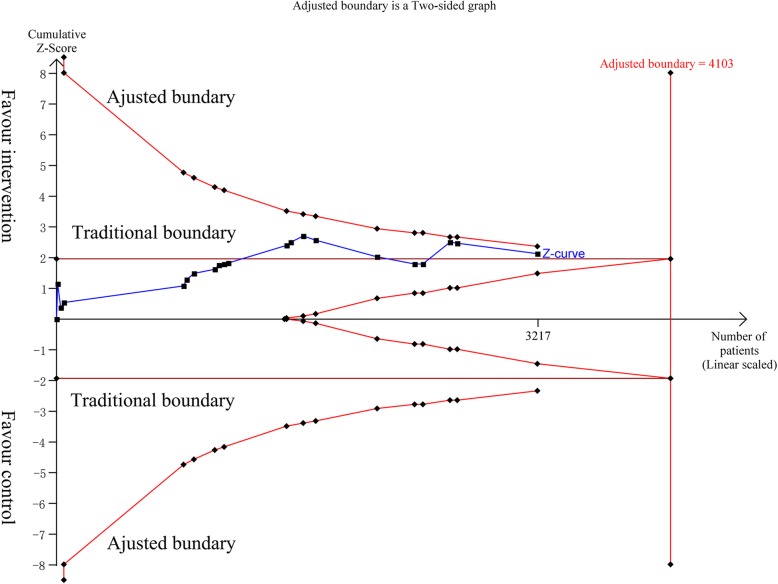
Fig. 3.
Trial sequential analysis for effects of vasopressin or its analogues on 28/30-day mortality. The diversity-adjusted required information size (4103 participants) was based on a relative risk reduction of 10%, an alpha of 5%, a beta of 20%, and an event proportion of 43% in the control arm. The blue cumulative z curve was constructed using a fixed effects model